Platelet activation and prothrombotic properties in a mouse model of peritoneal sepsis
- PMID: 30201980
- PMCID: PMC6131186
- DOI: 10.1038/s41598-018-31910-8
Platelet activation and prothrombotic properties in a mouse model of peritoneal sepsis
Abstract
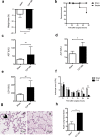
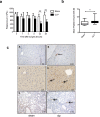
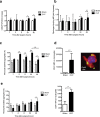
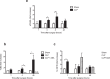
Sepsis is associated with thrombocytopenia and microvascular thrombosis. Studies have described platelets implication in this pathology but their kinetics of activation and behavior remain poorly known. We show in a mouse model of peritonitis, the appearance of platelet-rich thrombi in organ microvessels and organ damage. Complementary methods are necessary to characterize platelet activation during sepsis as circulating soluble markers and platelet-monocyte aggregates revealed early platelet activation, while surface activation markers were detected at later stage. A microfluidic based ex-vivo thrombosis assay demonstrated that platelets from septic mice have a prothrombotic behavior at shear rate encountered in microvessels. Interestingly, we found that even though phosphoinositide-3-kinase β-deficient platelet mice formed less thrombi in liver microcirculation, peritoneal sepsis activates a platelet alternative pathway to compensate the otherwise mandatory role of this lipid-kinase to form stable thrombi at high shear rate. Platelets are rapidly activated during sepsis. Thrombocytopenia can be attributed in part to platelet-rich thrombi formation in capillaries and platelet-leukocytes interactions. Platelets from septic mice have a prothrombotic phenotype at a shear rate encountered in arterioles. Further studies are necessary to unravel molecular mechanisms leading to this prothrombotic state of platelets in order to guide the development of future treatments of polymicrobial sepsis.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Prothrombotic factors enhance heparin-induced thrombocytopenia and thrombosis in vivo in a mouse model.J Thromb Haemost. 2006 Dec;4(12):2687-94. doi: 10.1111/j.1538-7836.2006.02201.x. Epub 2006 Sep 8. J Thromb Haemost. 2006. PMID: 16961586
-
Platelets Endocytose Viral Particles and Are Activated via TLR (Toll-Like Receptor) Signaling.Arterioscler Thromb Vasc Biol. 2020 Jul;40(7):1635-1650. doi: 10.1161/ATVBAHA.120.314180. Epub 2020 May 21. Arterioscler Thromb Vasc Biol. 2020. PMID: 32434410 Free PMC article.
-
Evolution of platelet activation parameters during septic shock in intensive care unit.Platelets. 2022 Aug 18;33(6):918-925. doi: 10.1080/09537104.2021.2007873. Epub 2021 Dec 16. Platelets. 2022. PMID: 34915822
-
Platelet function in sepsis.Crit Care Med. 2002 May;30(5 Suppl):S313-7. doi: 10.1097/00003246-200205001-00022. Crit Care Med. 2002. PMID: 12004253 Review.
-
The prothrombotic potential of platelet factor 4.Eur J Intern Med. 2010 Apr;21(2):79-83. doi: 10.1016/j.ejim.2009.11.007. Epub 2009 Dec 23. Eur J Intern Med. 2010. PMID: 20206875 Review.
Cited by
-
The Actin-Binding Protein Cortactin Promotes Sepsis Severity by Supporting Excessive Neutrophil Infiltration into the Lung.Biomedicines. 2022 Apr 28;10(5):1019. doi: 10.3390/biomedicines10051019. Biomedicines. 2022. PMID: 35625756 Free PMC article.
-
Platelets Are Critical Key Players in Sepsis.Int J Mol Sci. 2019 Jul 16;20(14):3494. doi: 10.3390/ijms20143494. Int J Mol Sci. 2019. PMID: 31315248 Free PMC article. Review.
-
Efficacy and Safety of Recombinant Human Thrombopoietin on Sepsis Patients With Thrombocytopenia: A Systematic Review and Meta-Analysis.Front Pharmacol. 2020 Jun 24;11:940. doi: 10.3389/fphar.2020.00940. eCollection 2020. Front Pharmacol. 2020. PMID: 32714186 Free PMC article.
-
Platelet specific knockout of integrin beta-3 (β3) reduces severity of necrotizing enterocolitis in murine neonates.Front Pediatr. 2025 Jul 17;13:1560242. doi: 10.3389/fped.2025.1560242. eCollection 2025. Front Pediatr. 2025. PMID: 40746354 Free PMC article.
-
Western Diet Modifies Platelet Activation Profiles in Male Mice.Int J Mol Sci. 2024 Jul 23;25(15):8019. doi: 10.3390/ijms25158019. Int J Mol Sci. 2024. PMID: 39125586 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

