New Optical Imaging Reporter-labeled Anaplastic Thyroid Cancer-Derived Extracellular Vesicles as a Platform for In Vivo Tumor Targeting in a Mouse Model
- PMID: 30201988
- PMCID: PMC6131173
- DOI: 10.1038/s41598-018-31998-y
New Optical Imaging Reporter-labeled Anaplastic Thyroid Cancer-Derived Extracellular Vesicles as a Platform for In Vivo Tumor Targeting in a Mouse Model
Abstract
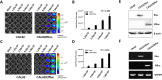
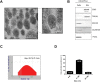
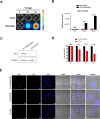
Extracellular vesicles (EVs), originating from multivesicular bodies by invagination of the endosomal membrane, are communication channels between distant cells. They are natural carriers of exogeneous cellular materials and have been exploited as drug delivery carriers in various diseases. Here, we found that tumor cell-derived EVs can be used as efficient targets in tumors by monitoring with an optical reporter system. Anaplastic thyroid cancer (CAL62) cell-derived EVs with Renilla luciferase (Rluc) were used to target CAL62 tumors in a mouse model. Optical imaging revealed that cancer cell-derived EVs (EV-CAL62/Rluc) targeted the original tumor (CAL62) in mice within 30 min after systemic injection. Furthermore, fluorescence imaging revealed that EV-CAL62/Rluc were internalized into CAL62 tumors in the mice. Ex vivo Optical imaging further confirmed the in vivo finding. Here, we successfully monitored the tumor targeting ability of tumor cell-derived EVs by optical imaging. Based on these results, tumor cell-derived EVs are highly effective natural carriers for drug delivery for cancer therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
In Vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model.Sci Rep. 2016 Jul 25;6:30418. doi: 10.1038/srep30418. Sci Rep. 2016. PMID: 27452924 Free PMC article.
-
Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin.Int J Med Sci. 2018 Jun 22;15(10):1051-1061. doi: 10.7150/ijms.25760. eCollection 2018. Int J Med Sci. 2018. PMID: 30013447 Free PMC article.
-
Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer.Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):7750-5. doi: 10.1073/pnas.1605841113. Epub 2016 Jun 24. Proc Natl Acad Sci U S A. 2016. PMID: 27342857 Free PMC article.
-
Membrane Derived Vesicles as Biomimetic Carriers for Targeted Drug Delivery System.Curr Top Med Chem. 2020;20(27):2472-2492. doi: 10.2174/1568026620666200922113054. Curr Top Med Chem. 2020. PMID: 32962615 Review.
-
Extracellular Vesicles - Advanced Nanocarriers in Cancer Therapy: Progress and Achievements.Int J Nanomedicine. 2020 Aug 26;15:6485-6502. doi: 10.2147/IJN.S238099. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32922012 Free PMC article. Review.
Cited by
-
CTC, ctDNA, and Exosome in Thyroid Cancers: A Review.Int J Mol Sci. 2023 Sep 6;24(18):13767. doi: 10.3390/ijms241813767. Int J Mol Sci. 2023. PMID: 37762070 Free PMC article. Review.
-
Radioiodine labeling and in vivo trafficking of extracellular vesicles.Sci Rep. 2021 Mar 3;11(1):5041. doi: 10.1038/s41598-021-84636-5. Sci Rep. 2021. PMID: 33658566 Free PMC article.
-
A Review of Labeling Approaches Used in Small Extracellular Vesicles Tracing and Imaging.Int J Nanomedicine. 2023 Aug 11;18:4567-4588. doi: 10.2147/IJN.S416131. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37588627 Free PMC article. Review.
-
Extracellular Vesicles as Mediators of Cancer Disease and as Nanosystems in Theranostic Applications.Cancers (Basel). 2021 Jul 2;13(13):3324. doi: 10.3390/cancers13133324. Cancers (Basel). 2021. PMID: 34283059 Free PMC article. Review.
-
The Role of Exosomes in Thyroid Cancer and Their Potential Clinical Application.Front Oncol. 2020 Dec 1;10:596132. doi: 10.3389/fonc.2020.596132. eCollection 2020. Front Oncol. 2020. PMID: 33335859 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

