CXCL12 and MYC control energy metabolism to support adaptive responses after kidney injury
- PMID: 30202007
- PMCID: PMC6131511
- DOI: 10.1038/s41467-018-06094-4
CXCL12 and MYC control energy metabolism to support adaptive responses after kidney injury
Abstract
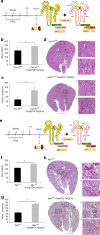
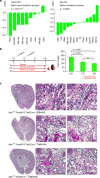
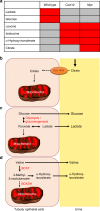
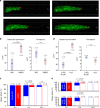
Kidney injury is a common complication of severe disease. Here, we report that injuries of the zebrafish embryonal kidney are rapidly repaired by a migratory response in 2-, but not in 1-day-old embryos. Gene expression profiles between these two developmental stages identify cxcl12a and myca as candidates involved in the repair process. Zebrafish embryos with cxcl12a, cxcr4b, or myca deficiency display repair abnormalities, confirming their role in response to injury. In mice with a kidney-specific knockout, Cxcl12 and Myc gene deletions suppress mitochondrial metabolism and glycolysis, and delay the recovery after ischemia/reperfusion injury. Probing these observations in zebrafish reveal that inhibition of glycolysis slows fast migrating cells and delays the repair after injury, but does not affect the slow cell movements during kidney development. Our findings demonstrate that Cxcl12 and Myc facilitate glycolysis to promote fast migratory responses during development and repair, and potentially also during tumor invasion and metastasis.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Nephron repair: powered by anaerobic energy metabolism.Ann Transl Med. 2019 Mar;7(Suppl 1):S28. doi: 10.21037/atm.2019.01.73. Ann Transl Med. 2019. PMID: 31032308 Free PMC article. No abstract available.
-
Mind the gap: renal tubule responses to injury and the role of Cxcl12 and Myc.Ann Transl Med. 2019 Mar;7(Suppl 1):S30. doi: 10.21037/atm.2019.01.80. Ann Transl Med. 2019. PMID: 31032310 Free PMC article. No abstract available.
Similar articles
-
CXCL12a/CXCR4b acts to retain neutrophils in caudal hematopoietic tissue and to antagonize recruitment to an injury site in the zebrafish larva.Immunogenetics. 2017 May;69(5):341-349. doi: 10.1007/s00251-017-0975-9. Epub 2017 Feb 20. Immunogenetics. 2017. PMID: 28220184
-
Tetraspanin Cd9b and Cxcl12a/Cxcr4b have a synergistic effect on the control of collective cell migration.PLoS One. 2021 Nov 30;16(11):e0260372. doi: 10.1371/journal.pone.0260372. eCollection 2021. PLoS One. 2021. PMID: 34847198 Free PMC article.
-
Involvement of Cxcl12a/Cxcr4b Chemokine System in Mediating the Stimulatory Effect of Embryonic Ethanol Exposure on Neuronal Density in Zebrafish Hypothalamus.Alcohol Clin Exp Res. 2020 Dec;44(12):2519-2535. doi: 10.1111/acer.14482. Epub 2020 Nov 16. Alcohol Clin Exp Res. 2020. PMID: 33067812 Free PMC article.
-
Gβ1 controls collective cell migration by regulating the protrusive activity of leader cells in the posterior lateral line primordium.Dev Biol. 2014 Jan 15;385(2):316-27. doi: 10.1016/j.ydbio.2013.10.027. Epub 2013 Nov 4. Dev Biol. 2014. PMID: 24201188 Free PMC article.
-
Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology.Curr Opin Nephrol Hypertens. 2011 Jul;20(4):416-24. doi: 10.1097/MNH.0b013e3283477797. Curr Opin Nephrol Hypertens. 2011. PMID: 21519251 Review.
Cited by
-
Histone Deacetylases Cooperate with NF-κB to Support the Immediate Migratory Response after Zebrafish Pronephros Injury.Int J Mol Sci. 2022 Aug 24;23(17):9582. doi: 10.3390/ijms23179582. Int J Mol Sci. 2022. PMID: 36076983 Free PMC article.
-
Hypoxic preconditioning protects against ischemic kidney injury through the IDO1/kynurenine pathway.Cell Rep. 2021 Aug 17;36(7):109547. doi: 10.1016/j.celrep.2021.109547. Cell Rep. 2021. PMID: 34407414 Free PMC article.
-
Nicotinamide Attenuates the Progression of Renal Failure in a Mouse Model of Adenine-Induced Chronic Kidney Disease.Toxins (Basel). 2021 Jan 11;13(1):50. doi: 10.3390/toxins13010050. Toxins (Basel). 2021. PMID: 33440677 Free PMC article.
-
Modeling Podocyte Ontogeny and Podocytopathies with the Zebrafish.J Dev Biol. 2023 Feb 20;11(1):9. doi: 10.3390/jdb11010009. J Dev Biol. 2023. PMID: 36810461 Free PMC article. Review.
-
Non-dialyzable uremic toxins and renal tubular cell damage in CKD patients: a systems biology approach.Eur J Med Res. 2024 Aug 9;29(1):412. doi: 10.1186/s40001-024-01951-z. Eur J Med Res. 2024. PMID: 39123228 Free PMC article.
References
-
- Drummond IA, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

