Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection
- PMID: 30202057
- PMCID: PMC6131390
- DOI: 10.1038/s41467-018-06103-6
Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection
Abstract
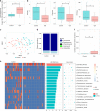
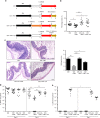
Fecal microbiota transplantation (FMT) is effective in treating recurrent Clostridium difficile infection (CDI). Bacterial colonization in recipients after FMT has been studied, but little is known about the role of the gut fungal community, or mycobiota. Here, we show evidence of gut fungal dysbiosis in CDI, and that donor-derived fungal colonization in recipients is associated with FMT response. CDI is accompanied by over-representation of Candida albicans and decreased fungal diversity, richness, and evenness. Cure after FMT is associated with increased colonization of donor-derived fungal taxa in recipients. Recipients of successful FMT ("responders") display, after FMT, a high relative abundance of Saccharomyces and Aspergillus, whereas "nonresponders" and individuals treated with antibiotics display a dominant presence of Candida. High abundance of C. albicans in donor stool also correlates with reduced FMT efficacy. Furthermore, C. albicans reduces FMT efficacy in a mouse model of CDI, while antifungal treatment reestablishes its efficacy, supporting a potential causal relationship between gut fungal dysbiosis and FMT outcome.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients.Transpl Infect Dis. 2016 Aug;18(4):628-33. doi: 10.1111/tid.12550. Epub 2016 Jul 5. Transpl Infect Dis. 2016. PMID: 27214585 Clinical Trial.
-
Longitudinal microbiome analysis of single donor fecal microbiota transplantation in patients with recurrent Clostridium difficile infection and/or ulcerative colitis.PLoS One. 2018 Jan 31;13(1):e0190997. doi: 10.1371/journal.pone.0190997. eCollection 2018. PLoS One. 2018. PMID: 29385143 Free PMC article.
-
Successful therapy of Clostridium difficile infection with fecal microbiota transplantation.J Physiol Pharmacol. 2016 Dec;67(6):859-866. J Physiol Pharmacol. 2016. PMID: 28195066
-
Fecal microbiota transplantation for the treatment of Clostridium difficile infection.J Hosp Med. 2016 Jan;11(1):56-61. doi: 10.1002/jhm.2449. Epub 2015 Sep 7. J Hosp Med. 2016. PMID: 26344412 Free PMC article. Review.
-
Fecal microbiota transplantation for Clostridium difficile infection: back to the future.Expert Opin Biol Ther. 2015 Jul;15(7):1001-14. doi: 10.1517/14712598.2015.1045872. Expert Opin Biol Ther. 2015. PMID: 26063385 Review.
Cited by
-
Global research trends and hotspots on human intestinal fungi and health: a bibliometric visualization study.Front Cell Infect Microbiol. 2024 Oct 17;14:1460570. doi: 10.3389/fcimb.2024.1460570. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39483119 Free PMC article.
-
Interactions between Candida albicans and the resident microbiota.Front Microbiol. 2022 Sep 20;13:930495. doi: 10.3389/fmicb.2022.930495. eCollection 2022. Front Microbiol. 2022. PMID: 36204612 Free PMC article. Review.
-
Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases.Int J Mol Sci. 2023 Oct 27;24(21):15654. doi: 10.3390/ijms242115654. Int J Mol Sci. 2023. PMID: 37958637 Free PMC article. Review.
-
A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors.Cell. 2022 Sep 29;185(20):3807-3822.e12. doi: 10.1016/j.cell.2022.09.015. Cell. 2022. PMID: 36179671 Free PMC article.
-
The microbiota: a crucial mediator in gut homeostasis and colonization resistance.Front Microbiol. 2024 Aug 6;15:1417864. doi: 10.3389/fmicb.2024.1417864. eCollection 2024. Front Microbiol. 2024. PMID: 39165572 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

