Generation and assembly of human brain region-specific three-dimensional cultures
- PMID: 30202107
- PMCID: PMC6597009
- DOI: 10.1038/s41596-018-0032-7
Generation and assembly of human brain region-specific three-dimensional cultures
Abstract
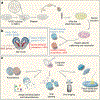
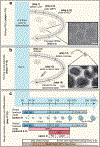
The ability to generate region-specific three-dimensional (3D) models to study human brain development offers great promise for understanding the nervous system in both healthy individuals and patients. In this protocol, we describe how to generate and assemble subdomain-specific forebrain spheroids, also known as brain region-specific organoids, from human pluripotent stem cells (hPSCs). We describe how to pattern the neural spheroids toward either a dorsal forebrain or a ventral forebrain fate, establishing human cortical spheroids (hCSs) and human subpallial spheroids (hSSs), respectively. We also describe how to combine the neural spheroids in vitro to assemble forebrain assembloids that recapitulate the interactions of glutamatergic and GABAergic neurons seen in vivo. Astrocytes are also present in the human forebrain-specific spheroids, and these undergo maturation when the forebrain spheroids are cultured long term. The initial generation of neural spheroids from hPSCs occurs in <1 week, with regional patterning occurring over the subsequent 5 weeks. After the maturation stage, brain region-specific spheroids are amenable to a variety of assays, including live-cell imaging, calcium dynamics, electrophysiology, cell purification, single-cell transcriptomics, and immunohistochemistry studies. Once generated, forebrain spheroids can also be matured for >24 months in culture.
Conflict of interest statement
CONFLICT OF INTEREST
The author declares no competing financial interests.
Figures


Similar articles
-
Assembly of functionally integrated human forebrain spheroids.Nature. 2017 May 4;545(7652):54-59. doi: 10.1038/nature22330. Epub 2017 Apr 26. Nature. 2017. PMID: 28445465 Free PMC article.
-
Engineering brain assembloids to interrogate human neural circuits.Nat Protoc. 2022 Jan;17(1):15-35. doi: 10.1038/s41596-021-00632-z. Epub 2022 Jan 6. Nat Protoc. 2022. PMID: 34992269 Review.
-
Method to Generate Dorsal Forebrain Brain Organoids from Human Pluripotent Stem Cells.Methods Mol Biol. 2023;2683:169-183. doi: 10.1007/978-1-0716-3287-1_13. Methods Mol Biol. 2023. PMID: 37300774
-
The rise of three-dimensional human brain cultures.Nature. 2018 Jan 24;553(7689):437-445. doi: 10.1038/nature25032. Nature. 2018. PMID: 29364288 Review.
-
Guided self-organization and cortical plate formation in human brain organoids.Nat Biotechnol. 2017 Jul;35(7):659-666. doi: 10.1038/nbt.3906. Epub 2017 May 31. Nat Biotechnol. 2017. PMID: 28562594 Free PMC article.
Cited by
-
Human cerebral organoids: cellular composition and subcellular morphological features.Front Cell Neurosci. 2024 Jun 12;18:1406839. doi: 10.3389/fncel.2024.1406839. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38933177 Free PMC article.
-
Patterning Neuroepithelial Cell Sheet via a Sustained Chemical Gradient Generated by Localized Passive Diffusion Devices.ACS Biomater Sci Eng. 2021 Apr 12;7(4):1713-1721. doi: 10.1021/acsbiomaterials.0c01365. Epub 2021 Mar 22. ACS Biomater Sci Eng. 2021. PMID: 33751893 Free PMC article.
-
Microfluidics for Neuronal Cell and Circuit Engineering.Chem Rev. 2022 Sep 28;122(18):14842-14880. doi: 10.1021/acs.chemrev.2c00212. Epub 2022 Sep 7. Chem Rev. 2022. PMID: 36070858 Free PMC article. Review.
-
Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy.Biomedicines. 2022 Apr 19;10(5):937. doi: 10.3390/biomedicines10050937. Biomedicines. 2022. PMID: 35625674 Free PMC article. Review.
-
Neurodevelopmental copy-number variants: A roadmap to improving outcomes by uniting patient advocates, researchers, and clinicians for collective impact.Am J Hum Genet. 2022 Aug 4;109(8):1353-1365. doi: 10.1016/j.ajhg.2022.07.003. Am J Hum Genet. 2022. PMID: 35931048 Free PMC article. Review.
References
-
- Takahashi K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872, doi: (2007). - PubMed
-
- Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676, doi: (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

