Efficacy and Safety of Valsartan or Chlorthalidone vs. Combined Valsartan and Chlorthalidone in Patients With Mild to Moderate Hypertension: The VACLOR Study
- PMID: 30202211
- PMCID: PMC6122245
- DOI: 10.1177/1179546818796482
Efficacy and Safety of Valsartan or Chlorthalidone vs. Combined Valsartan and Chlorthalidone in Patients With Mild to Moderate Hypertension: The VACLOR Study
Abstract
Objective: To evaluate the efficacy and safety of valsartan (V) or chlorthalidone (C) monotherapy in comparison with a fixed combination of valsartan and chlorthalidone (V + C).
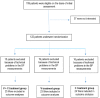
Methods: This 12-week multicenter randomized three-arm open-label study randomly allocated 72 patients to V or C as monotherapy or a combination of V + C. The aim was to measure changes in office systolic blood pressure (SBP) and diastolic blood pressure (DBP) and in 24-hour ambulatory blood pressure monitoring (ABPM) from baseline to week 12, in addition to medication tolerability.
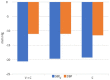
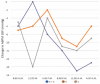
Results: The proportion of patients achieving target BP in office at week 12 was not statistically different for the three groups. However, comparisons of daytime and nighttime 24-hour ABPM values from baseline to week 12 revealed significant differences in nighttime mean SBP for the three groups, due to a significantly greater reduction in the values in patients assigned to the V + C group (-14.7 vs. -8.7 vs. -10.7, P = .042, V+C; V; C, respectively). Although patients assigned to the V + C group also had greater nighttime reduction in mean DBP values compared with those in the other groups, this difference was not statistically significant. The incidence of adverse events did not differ significantly.
Conclusion: In patients with hypertension treated with V, C, and both medications combined, the fixed combination of V + C provided a significantly greater reduction of late night to early morning BP values when interventions were assessed with 24-hour ABPM.
Trial registration: clinicaltrials.gov Identifier: NCT.01850160, https://clinicaltrials.gov/ct2/show/NCT01850160.
Keywords: Hypertension; chlorthalidone; combined therapy; monotherapy; valsartan.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Juan Romero is a full-time employee of Farma. Dr. Carlos Rodriguez-Martinez has received partial compensation for statistical analysis. Data entry and statistical analyses were supported by Farma. Dr. Manzur and Dr. Rico report no potential conflict of interest relevant to this article.
Figures


Similar articles
-
Effects of combination olmesartan medoxomil plus azelnidipine versus monotherapy with either agent on 24-hour ambulatory blood pressure and pulse rate in Japanese patients with essential hypertension: additional results from the REZALT study.Clin Ther. 2010 May;32(5):861-81. doi: 10.1016/j.clinthera.2010.04.020. Clin Ther. 2010. PMID: 20685495 Clinical Trial.
-
Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: the VAST study.Clin Ther. 2005 May;27(5):578-87. doi: 10.1016/j.clinthera.2005.05.006. Clin Ther. 2005. PMID: 15978306 Clinical Trial.
-
Ambulatory blood pressure response to once-daily fimasartan: an 8-week, multicenter, randomized, double-blind, active-comparator, parallel-group study in Korean patients with mild to moderate essential hypertension.Clin Ther. 2013 Sep;35(9):1337-49. doi: 10.1016/j.clinthera.2013.06.021. Epub 2013 Aug 7. Clin Ther. 2013. PMID: 23932463 Clinical Trial.
-
Evaluation of the Efficacy and Safety of the Lercanidipine/Valsartan Combination in Korean Patients With Essential Hypertension Not Adequately Controlled With Lercanidipine Monotherapy: A Randomized, Multicenter, Parallel Design, Phase III Clinical Trial.Clin Ther. 2015 Aug;37(8):1726-39. doi: 10.1016/j.clinthera.2015.05.512. Epub 2015 Jul 8. Clin Ther. 2015. PMID: 26164786 Clinical Trial.
-
Azilsartan medoxomil: a review of its use in hypertension.Clin Drug Investig. 2012 Sep 1;32(9):621-39. doi: 10.2165/11209600-000000000-00000. Clin Drug Investig. 2012. PMID: 22877322 Review.
Cited by
-
Efficacy of angiotensin receptor blockers for nocturnal blood pressure reduction: a systematic review and meta-analysis.Ann Med. 2024 Dec;56(1):2362880. doi: 10.1080/07853890.2024.2362880. Epub 2024 Jun 3. Ann Med. 2024. PMID: 38830046 Free PMC article.
-
Head-out immersion in natural thermal mineral water for the management of hypertension: a review of randomized controlled trials.Int J Biometeorol. 2019 Dec;63(12):1707-1718. doi: 10.1007/s00484-019-01780-4. Epub 2019 Aug 11. Int J Biometeorol. 2019. PMID: 31402400 Review.
References
-
- Forouzanfar M, Alexander L, Anderson H, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. 10.1016/s0140-6736(15)00128-2. - DOI - PMC - PubMed
-
- Informe técnico: Carga de enfermedad por enfermedades crónicas no transmisibles y discapacidad en Colombia. Instituto Nacional de Salud. 2015. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/IA/IN.... Consultado September 8, 2017.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

