A comprehensive review on Brigatinib - A wonder drug for targeted cancer therapy in non-small cell lung cancer
- PMID: 30202213
- PMCID: PMC6128722
- DOI: 10.1016/j.jsps.2018.04.010
A comprehensive review on Brigatinib - A wonder drug for targeted cancer therapy in non-small cell lung cancer
Abstract
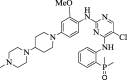
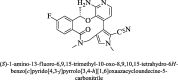
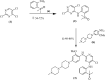
The mortality rate in patients suffering from non-small cell lung cancer (NSCLC) is quite high. This type of cancer mainly occurs due to rearrangements in the anaplastic lymphoma kinase (ALK) gene which leads to form an oncogene of fused gene NPM-ALK. Brigatinib is recently approved by FDA in April 2017 as a potent tyrosine kinase inhibitor (TKI) for the NSCLC therapy. In the present scenario, it is no less than a wonder drug because it is indicated for the treatment of advanced stages of metastatic ALK positive NSCLC, a fatal disease to overcome the resistance of various other ALK inhibitors such as crizotinib, ceritinib and alectinib. In addition to ALK, it is also active against multiple types of kinases such as ROS1, Insulin like growth factor-1Receptor and EGFR. It can be synthesized by using N-[2-methoxy-4-[4-(dimethylamino) piperidin-1-yl] aniline] guanidine and 2,4,5-trichloropyrimidine respectively in two different ways. Its structure consists of mainly dimethylphosphine oxide group which is responsible for its pharmacological activity. It is active against various cell lines such as HCC78, H2228, H23, H358, H838, U937, HepG2 and Karpas- 299. Results of ALTA (ALK in Lung Cancer Trial of AP26113) phase ½ trial shows that 90 mg of brigatinib for 7 days and then 180 mg for next days is effective in the treatment of NSCLC. Brigatinib has been shown to have favorable risk benefit profile and is a safer drug than the available cytotoxic chemotherapeutic agents. In comparison to other FDA approved drugs for the same condition, it causes fewer minor adverse reactions which can be easily managed either by changing the dose or by providing good supportive care. This article is intended to provide readers with an overview of chemistry, pharmacokinetic, pharmacodynamic and safety profile of brigatinib, which addresses an unmet medical need.
Keywords: ALCL, anaplastic extensive cell lymphoma; ALK inhibitors; ALK, anaplastic lymphoma kinase; ALTA-1L, ALK in lung cancer trial of Brigatinib in1st Line; BCRP, breast cancer resistance protein; Brigatinib; DMPO, dimethyl phosphine oxide; EGFR, epidermal growth factor receptor; EML4, echinoderm microtubule associated protein; FDA, Food and Drug Administration; FLT3, fem like tyrosine kinase-3; Kinase; LCC, Large Cell Carcinoma; Lung cancer; Lymphoma; MIC, minimum inhibitory concentration; NPM, nucleophosmin; NSCLC, non-small cell lung cancer; ORR, objective response rate; P-gp, P-glycoprotein; SAR, structure activity relationship; TKI’s, tyrosine kinase inhibitors.
Figures
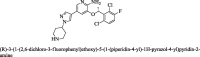

Similar articles
-
ALTA-2: Phase II study of brigatinib in patients with ALK-positive, advanced non-small-cell lung cancer who progressed on alectinib or ceritinib.Future Oncol. 2021 May;17(14):1709-1719. doi: 10.2217/fon-2020-1119. Epub 2021 Feb 11. Future Oncol. 2021. PMID: 33569983
-
P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer.EBioMedicine. 2015 Dec 12;3:54-66. doi: 10.1016/j.ebiom.2015.12.009. eCollection 2016 Jan. EBioMedicine. 2015. PMID: 26870817 Free PMC article.
-
Efficacy of Brigatinib in Patients With Advanced ALK-Positive NSCLC Who Progressed on Alectinib or Ceritinib: ALK in Lung Cancer Trial of brigAtinib-2 (ALTA-2).J Thorac Oncol. 2022 Dec;17(12):1404-1414. doi: 10.1016/j.jtho.2022.08.018. Epub 2022 Sep 10. J Thorac Oncol. 2022. PMID: 36096442
-
Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors.Expert Rev Anticancer Ther. 2018 Jan;18(1):71-80. doi: 10.1080/14737140.2018.1412260. Epub 2017 Dec 6. Expert Rev Anticancer Ther. 2018. PMID: 29187012 Review.
-
Strengths and pitfalls of brigatinib in non-small cell lung cancer patients' management.Minerva Med. 2022 Apr;113(2):315-332. doi: 10.23736/S0026-4806.21.07693-X. Minerva Med. 2022. PMID: 35575153 Review.
Cited by
-
An insight into lung cancer: a comprehensive review exploring ALK TKI and mechanisms of resistance.Bosn J Basic Med Sci. 2022 Feb 1;22(1):1-13. doi: 10.17305/bjbms.2021.5859. Bosn J Basic Med Sci. 2022. PMID: 34082691 Free PMC article. Review.
-
Microwave-assisted multicomponent synthesis of antiproliferative 2,4-dimethoxy-tetrahydropyrimido[4,5-b]quinolin-6(7H)-ones.RSC Adv. 2022 Oct 25;12(47):30404-30415. doi: 10.1039/d2ra04669e. eCollection 2022 Oct 24. RSC Adv. 2022. PMID: 36337956 Free PMC article.
-
Drug-Related Pneumonitis in Cancer Treatment during the COVID-19 Era.Cancers (Basel). 2021 Mar 2;13(5):1052. doi: 10.3390/cancers13051052. Cancers (Basel). 2021. PMID: 33801385 Free PMC article. Review.
-
Refractory Anaplastic Large Cell Lymphoma Rescued by the Combination of the Second-Generation ALK Inhibitor Brigatinib, High-dose Chemotherapy and Allogeneic Stem Cell Transplantation: A Case Report and Review of the Literature.Clin Hematol Int. 2023 Jun;5(2-3):130-138. doi: 10.1007/s44228-023-00038-6. Epub 2023 Apr 18. Clin Hematol Int. 2023. PMID: 37072555 Free PMC article. Review.
-
Safety and Tolerability of Anaplastic Lymphoma Kinase Inhibitors in Non-Small-Cell Lung Cancer.Drug Saf. 2019 Feb;42(2):199-209. doi: 10.1007/s40264-018-0771-y. Drug Saf. 2019. PMID: 30649741 Review.
References
-
- ARIAD Pharmaceuticals Inc., 2017. ALUNBRIGTM (brigatinib): US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208772lbl.pdf (accessed 14.02.18).
-
- Camidge D.R., Bazhenova L., Salgia R., Langer C.J., Gold K.A., Rosell R., Shaw A.T., Weiss G.J., Narasimhan N.I., Dorer D.J., Rivera V.M. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non–small cell lung cancer (NSCLC) J. Clin. Oncol. 2015;33(15):8062.
-
- Camidge D.R., Pao W., Sequist L.V. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev.Clin. Oncol. 2014;11(8):473–481. - PubMed
-
- Camidge D.R., Tiseo M., Ahn M.J., Reckamp K.L., Hansen K.H., Kim S.W., Huber R.M., West H.L., Groen H.J., Hochmair M.J., Leighl N.B. Brigatinib in crizotinib-refractory ALK+ NSCLC: Central assessment and updates from ALTA, a pivotal randomized phase 2 trial. J. Thorac. Oncol. 2017;12(1):1167–1169.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

