Evaluation of teratogenicity and genotoxicity induced by kramecyne (KACY)
- PMID: 30202224
- PMCID: PMC6128725
- DOI: 10.1016/j.jsps.2018.03.016
Evaluation of teratogenicity and genotoxicity induced by kramecyne (KACY)
Abstract
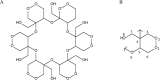
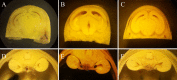
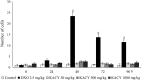
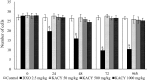
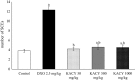
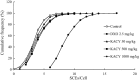
Kramecyne (KACY), a polymer isolated from Krameria cytisoides Cav, has anti-inflammatory, anti-nociceptive, anti-arthritic and anti-ulcerogenic properties. As a part of standard preclinical safety tests, the present study sought to determine potential developmental toxicity (in female rats) and genotoxicity (in male mice) of KACY. Pregnant female rats were divided into six groups: the negative control (vehicle), the positive control (250 mg/kg of acetylsalicylic acid (ASA)), and four experimental groups (50, 250, 500 and 1000 mg/kg of KACY). To evaluate genotoxicity by in vivo micronuclei (MN) and sister chromatid exchange (SCE) tests, male mice were divided into five groups: the negative control (vehicle), the positive control (1.5 and 2.5 mg/kg of doxorubicin for MN and SCE, respectively), and three experimental groups (50, 500 and 1000 mg/kg of KACY). All treatments were administered by oral gavage. A slight maternal toxicity was evidenced by lower weight gain for rats receiving 500 and 1000 mg/kg of KACY, but no fetal malformations were found. However, there were less live fetuses/litter and greater post-implantation loss/litter at these two doses. Manifestations of developmental toxicity were limited to a higher rate of skeletal alterations. The MN tests did not evidence genotoxicity or cytotoxicity. KACY caused a slightly but significantly increased frequency of SCE. Although KACY-treated rats had skeletal alterations, these apparently were not caused by a mechanism of genotoxicity. Furthermore, the same administration in adult male mice did not produce genotoxicity. Hence, KACY herein proved to be safe for rats during the period of organogenesis.
Keywords: Kramecyne; Micronuclei; Sister chromatid exchange; Teratogenic study.
Figures





Similar articles
-
Antinociceptive and anti-arthritic effects of kramecyne.Life Sci. 2015 Jan 15;121:70-7. doi: 10.1016/j.lfs.2014.11.015. Epub 2014 Dec 2. Life Sci. 2015. PMID: 25476830
-
Effects of kramecyne on LPS induced chronic inflammation and gastric ulcers.Drug Dev Res. 2015 Jun;76(4):185-93. doi: 10.1002/ddr.21254. Drug Dev Res. 2015. PMID: 26109468
-
NTP Toxicology and Carcinogenesis Studies of Theophylline (CAS No. 58-55-9) in F344/N Rats and B6C3F1 Mice (Feed and Gavage Studies).Natl Toxicol Program Tech Rep Ser. 1998 Aug;473:1-326. Natl Toxicol Program Tech Rep Ser. 1998. PMID: 12571677
-
Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin.Int J Toxicol. 2007;26 Suppl 1:3-106. doi: 10.1080/10915810601163939. Int J Toxicol. 2007. PMID: 17365137 Review.
-
Final report on the safety assessment of Triethylene Glycol and PEG-4.Int J Toxicol. 2006;25 Suppl 2:121-38. doi: 10.1080/10915810600964642. Int J Toxicol. 2006. PMID: 17090481 Review.
Cited by
-
Protective Effects of Phycobiliproteins from Arthrospira maxima (Spirulina) Against Cyclophosphamide-Induced Embryotoxicity and Genotoxicity in Pregnant CD1 Mice.Pharmaceuticals (Basel). 2025 Jan 15;18(1):101. doi: 10.3390/ph18010101. Pharmaceuticals (Basel). 2025. PMID: 39861163 Free PMC article.
-
Genotoxicity and repair capability of Mus musculus DNA following the oral exposure to Tramadol.Saudi J Biol Sci. 2020 Jan;27(1):12-17. doi: 10.1016/j.sjbs.2019.03.008. Epub 2019 Mar 26. Saudi J Biol Sci. 2020. PMID: 31889811 Free PMC article.
-
A Complete Review of Mexican Plants with Teratogenic Effects.Plants (Basel). 2022 Jun 24;11(13):1675. doi: 10.3390/plants11131675. Plants (Basel). 2022. PMID: 35807626 Free PMC article. Review.
References
-
- Alonso A.J., Zavala M.A., Pérez J., Sánchez S., Pérez S. Antinociceptive and anti-arthritic effects of kramecyne. Life Sci. 2015;121:70–77. - PubMed
-
- Alonso A.J., Pérez J., Sánchez E., Pérez C., Pérez S. Effects of kramecyne on LPS induced chronic inflammation and gastric ulcers. Drug Dev. Res. 2015;76:185–193. - PubMed
-
- Brambilla G., Cavanna M., Faggin P., Maura A., Pino A., Ricci R., Robbiano L. Genotoxic effects in rodents given high oral doses of ranitidine and sodium nitrite. Carcinogenesis. 1983;4(10):1281–1285. - PubMed
-
- Burdan F., Bełzek A. Current opinions on embryotoxic and teratogenic effects of ibuprofen. Pol. Merkur. Lekarski. 2001;11(63):266–270. - PubMed
-
- Cleves M.A., Savell V.H., Raj S., Zhao W., Correa A., Werler M.M., Hobbs C.A. Maternal use of acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), and muscular ventricular septal defects. Birth Defects Res. A. Clin. Mol. Teratol. 2004;70:107–113. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

