Synthesis, biological evaluation and in silico molecular docking of novel 1-hydroxy-naphthyl substituted heterocycles
- PMID: 30202227
- PMCID: PMC6128714
- DOI: 10.1016/j.jsps.2018.03.013
Synthesis, biological evaluation and in silico molecular docking of novel 1-hydroxy-naphthyl substituted heterocycles
Abstract
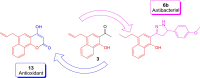
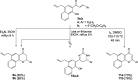
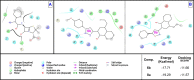
The versatile precursor 2-acetyl-4-allyl-1-hydroxy naphthalene was synthesized efficiently via Claisen rearrangement 2-acetyl-1-allyloxynaphthalene. The Claisen-Schmidt condensation of latter precursor afforded the corresponding chalcones which were exploited to synthesize a series of potential heterocycles such as pyrazoline, isoxazoline, benzocoumarin and benzoflavone. The synthesized products showed potent antioxidant and antimicrobial activities. Chalcone 3c, naphthyl pyrazoline 6b and hydroxycoumarin 13 exhibited the highest activity as antioxidants. Their binding mode showed specialized recognition of hydroxycoumarin 13 with the triad key amino acids at the active site of the oxidoreductase enzyme (PDB code 1DXO). 1-Hydroxynaphth-2-yl pyrazoline (6b) revealed the highest efficacy against both Gram positive and negative bacterial species. In silico molecular docking of pyrazoline 6b endorsed its proper binding at the active site of the 2EX6 enzyme which explains its potent antibacterial activity in comparison with standard ampicillin.
Keywords: 1DXO active site; Biological; Docking; Hydroxyacetyl, heterocycle; Naphthyl.
Figures





Similar articles
-
Design, synthesis, molecular modelling and biological evaluation of novel 3-(2-naphthyl)-1-phenyl-1H-pyrazole derivatives as potent antioxidants and 15-Lipoxygenase inhibitors.J Enzyme Inhib Med Chem. 2020 Dec;35(1):847-863. doi: 10.1080/14756366.2020.1742116. J Enzyme Inhib Med Chem. 2020. PMID: 32216479 Free PMC article.
-
Exploring the Antioxidant Potency of New Naphthalene-Based Chalcone Derivatives: Design, Synthesis, Antioxidant Evaluation, Docking Study, DFT Calculations.Chem Biodivers. 2023 Dec;20(12):e202301344. doi: 10.1002/cbdv.202301344. Epub 2023 Nov 22. Chem Biodivers. 2023. PMID: 37909089
-
Design, Synthesis, Antimicrobial Properties, and Molecular Docking of Novel Furan-Derived Chalcones and Their 3,5-Diaryl-∆2-pyrazoline Derivatives.Antibiotics (Basel). 2023 Dec 24;13(1):21. doi: 10.3390/antibiotics13010021. Antibiotics (Basel). 2023. PMID: 38247580 Free PMC article.
-
Synthesis and biological evaluation of chalcone derivatives (mini review).Mini Rev Med Chem. 2012 Nov;12(13):1394-403. doi: 10.2174/13895575112091394. Mini Rev Med Chem. 2012. PMID: 22876958 Review.
-
Recent developments in biological aspects of chalcones: the odyssey continues.Expert Opin Drug Discov. 2019 Mar;14(3):249-288. doi: 10.1080/17460441.2019.1573812. Expert Opin Drug Discov. 2019. PMID: 30773996 Review.
Cited by
-
Crystal structure, Hirshfeld surface analysis, and DFT and mol-ecular docking studies of 6-cyanona-phthalen-2-yl 4-(benz-yloxy)benzoate.Acta Crystallogr E Crystallogr Commun. 2024 Oct 22;80(Pt 11):1180-1185. doi: 10.1107/S2056989024009964. eCollection 2024 Oct 1. Acta Crystallogr E Crystallogr Commun. 2024. PMID: 39712165 Free PMC article.
-
Rigid 3D-spiro chromanone as a crux for efficient antimicrobial agents: synthesis, biological and computational evaluation.RSC Adv. 2021 Jun 16;11(35):21301-21314. doi: 10.1039/d1ra03497a. eCollection 2021 Jun 15. RSC Adv. 2021. PMID: 35478839 Free PMC article.
-
Synthesis of novel naphthalene-heterocycle hybrids with potent antitumor, anti-inflammatory and antituberculosis activities.RSC Adv. 2020 Nov 26;10(70):42998-43009. doi: 10.1039/d0ra08526j. eCollection 2020 Nov 23. RSC Adv. 2020. PMID: 35514936 Free PMC article.
-
Antibacterial potential of chalcones and its derivatives against Staphylococcus aureus.3 Biotech. 2023 Jan;13(1):1. doi: 10.1007/s13205-022-03398-7. Epub 2022 Dec 1. 3 Biotech. 2023. PMID: 36466769 Free PMC article. Review.
-
Naphthyl bearing 1,3,4-thiadiazoleacetamides targeting the parasitic folate pathway as anti-infectious agents: in silico, synthesis, and biological approach.RSC Med Chem. 2023 Nov 21;14(12):2768-2781. doi: 10.1039/d3md00423f. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38107179 Free PMC article.
References
-
- Abdel-Aziz A.A.-M., Abou-Zeid L.A., ElTahir K.E.H., Ayyad R.R., El-Sayed M.A.-A., El-Azab A.S. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4 (3H)-quinazolinones. Europ. J. Med. Chem. 2016;121:410–421. - PubMed
-
- Abdel-Rahman A.H., Hammouda M.A.A., El-Desoky S.I. Synthesis of some new azole, azepine, pyridine, and pyrimidine derivatives using 6-hydroxy-4H-4-oxo[1]-benzopyran-3-carboxaldehyde as a versatile starting material. Heteroat. Chem. 2005;16:20–27.
-
- Al-Omary F.A.M., Abou-zeid L.A., Nagi M.N., Habib S.E., Abdel-Aziz A.A.-M., El-Azab A.S., Abdel-Hamid S.G., Al-Omar M.A., Al-Obaid A.M., El-Subbagh H.I. Non-classical antifolates. Part 2: Synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg. Med. Chem. 2010;18:2849–2863. - PubMed
-
- Anto R.J., Sukumaran K., Kuttan G., Rao M.N.A., Subbaraju V., Kuttan R. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett. 1995;97:33–37. - PubMed
-
- Aziz G., Nosseir M.H., Doss N.L., Rizk A.S. Experiments with furochalcones: Addition of nucleophilic reagents, diazomethane, benzonitrile oxide, hydrazine hydrate, phenylhydrazine, hydroxylamine, thiourea and semicarbazide to furochalcones. Indian J. Chem. Sect. B. 1976;14:286–291.
LinkOut - more resources
Full Text Sources
Other Literature Sources

