Systematic review and meta-analysis on efficacy of cefixime for treating gonococcal infections
- PMID: 30202413
- PMCID: PMC6124830
Systematic review and meta-analysis on efficacy of cefixime for treating gonococcal infections
Abstract
Background: Neisseria gonorrhea is known to have developed a high level of resistance against different classes of antimicrobials. Patients with coagulation disorders where intramuscular injections are contraindicated, oral cefixime in combination therapy can be utilized as an alternative regimen. Cefixime in combination with another macrolide might be considered as an alternative treatment option. The aim of this systematic review is to assess the efficacy of 400 mg cefixime against a range of comparator drugs.
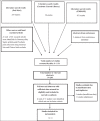
Methodology: Extensive literature search for randomized controlled trials was performed using Medline, Cochrane Registry of Controlled Trials, Embase, and Clinical trials registers. The trials assessed the efficacy of cefixime against a range of comparator drugs. Primary outcome of the study was the clinical resolution of signs and symptoms and negative culture at the end of follow-up period.
Results: After screening for a total of 1184, only 8 studies were eligible for a meta-analysis. Risk ratio random effects model was used with a 95% confidence interval (CI). The pooled efficacy of Cefixime was at 97% at 95 CI 1.01 (0.98, 1.05). No statistically significant difference was found between oral cefixime and comparator drugs.
Conclusion: A total of 11 studies were included following a review of 1184 publications. 8 randomized controlled trials for 400 mg oral cefixime were included in meta-analysis. Despite a high grade of evidence, a high risk of bias was found among studies. Hence, more high quality randomized controlled trials on cefixime needs to be performed in future to guide the treatment of gonococcal infections.
Keywords: Cefixime; Neisseria gonorrhea; efficacy; meta-analysis; sexually transmitted disease; sexually transmitted infections; systematic review.
Figures
References
-
- Datta SD, Sternberg M, Johnson RE, Berman S, Papp JR, McQuillan G, et al. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med. 2007;147:89–96. - PubMed
-
- Workowski KA, Berman SM, Douglas JM., Jr Emerging antimicrobial resistance inNeisseria gonorrhoeae:Urgent need to strengthen prevention strategies. Ann Intern Med. 2008;148:606–13. - PubMed
-
- Lind I. Antimicrobial resistance inNeisseria gonorrhoeae. Clin Infect Dis. 1997;24(Suppl 1):S93–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources