Bacterial conversion of depolymerized Kraft lignin
- PMID: 30202435
- PMCID: PMC6123935
- DOI: 10.1186/s13068-018-1240-7
Bacterial conversion of depolymerized Kraft lignin
Retraction in
-
Retraction Note to: Bacterial conversion of depolymerized Kraft lignin.Biotechnol Biofuels. 2018 Nov 12;11:313. doi: 10.1186/s13068-018-1307-5. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 30473731 Free PMC article.
Abstract
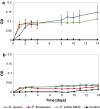
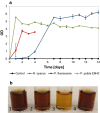
Background: Lignin is a potential feedstock for microbial conversion into various chemicals. However, the degradation rate of native or technical lignin is low, and depolymerization is needed to obtain reasonable conversion rates. In the current study, base-catalyzed depolymerization-using NaOH (5 wt%)-of softwood Kraft lignin was conducted in a continuous-flow reactor system at temperatures in the range 190-240 °C and residence times of 1 or 2 min. The ability of growth of nine bacterial strains belonging to the genera Pseudomonas and Rhodococcus was tested using the alkaline-treated lignin as a sole carbon source.
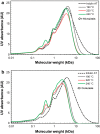
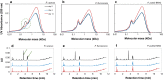
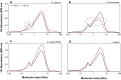
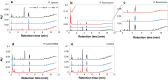
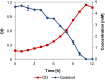
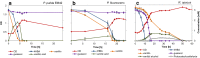
Results: Pseudomonas fluorescens and Rhodococcus opacus showed the best growth of the tested species on plates with lignin. Further evaluation of P. fluorescens and R. opacus was made in liquid cultivations with depolymerized lignin (DL) at a concentration of 1 g/L. Size exclusion chromatography (SEC) showed that R. opacus consumed most of the available lower molecular weight compounds (approximately 0.1-0.4 kDa) in the DL, but the weight distribution of larger fractions was almost unaffected. Importantly, the consumed compounds included guaiacol-one of the main monomers in the DL. SEC analysis of P. fluorescens culture broth, in contrast, did not show a large conversion of low molecular weight compounds, and guaiacol remained unconsumed. However, a significant shift in molecular weight distribution towards lower average weights was seen.
Conclusions: Rhodococcus opacus and P. fluorescens were identified as two potential microbial candidates for the conversion/consumption of base-catalyzed depolymerized lignin, acting on low and high molecular weight lignin fragments, respectively. These findings will be of relevance for designing bioconversion of softwood Kraft lignin.
Keywords: Base-catalyzed depolymerization; Biorefineries; Guaiacol; Indulin AT; Microbial conversion; Pseudomonas; Rhodococcus.
Figures
Similar articles
-
Bacterial conversion of depolymerized Kraft lignin.Biotechnol Biofuels. 2019 Mar 16;12:56. doi: 10.1186/s13068-019-1397-8. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 30923564 Free PMC article.
-
Membrane Separation of the Base-Catalyzed Depolymerization of Black Liquor Retentate for Low-Molecular-Mass Compound Production.Membranes (Basel). 2019 Aug 16;9(8):102. doi: 10.3390/membranes9080102. Membranes (Basel). 2019. PMID: 31426318 Free PMC article.
-
Development of Rhodococcus opacus as a chassis for lignin valorization and bioproduction of high-value compounds.Biotechnol Biofuels. 2019 Aug 5;12:192. doi: 10.1186/s13068-019-1535-3. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31404385 Free PMC article. Review.
-
A key O-demethylase in the degradation of guaiacol by Rhodococcus opacus PD630.Appl Environ Microbiol. 2023 Oct 31;89(10):e0052223. doi: 10.1128/aem.00522-23. Epub 2023 Oct 6. Appl Environ Microbiol. 2023. PMID: 37800939 Free PMC article.
-
Bioconversion of renewable feedstocks by Rhodococcus opacus.Curr Opin Biotechnol. 2020 Aug;64:10-16. doi: 10.1016/j.copbio.2019.08.013. Epub 2019 Sep 30. Curr Opin Biotechnol. 2020. PMID: 31580993 Review.
Cited by
-
Retraction Note to: Bacterial conversion of depolymerized Kraft lignin.Biotechnol Biofuels. 2018 Nov 12;11:313. doi: 10.1186/s13068-018-1307-5. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 30473731 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

