Applications of organocatalysed visible-light photoredox reactions for medicinal chemistry
- PMID: 30202458
- PMCID: PMC6122060
- DOI: 10.3762/bjoc.14.179
Applications of organocatalysed visible-light photoredox reactions for medicinal chemistry
Abstract
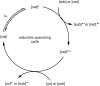
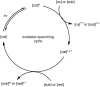
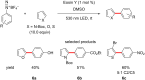
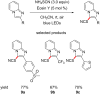
The focus of this review is to provide an overview of the field of organocatalysed photoredox chemistry relevant to synthetic medicinal chemistry. Photoredox transformations have been shown to enable key transformations that are important to the pharmaceutical industry. This type of chemistry has also demonstrated a high degree of sustainability, especially when organic dyes can be employed in place of often toxic and environmentally damaging transition metals. The sections are arranged according to the general class of the presented reactions and the value of these methods to medicinal chemistry is considered. An overview of the general characteristics of the photocatalysts as well as some electrochemical data is presented. In addition, the general reaction mechanisms for organocatalysed photoredox transformations are discussed and some individual mechanistic considerations are highlighted in the text when appropriate.
Keywords: C–H functionalisation; heterocycles; late-stage functionalisation; medicinal chemistry; organic dyes; organic photocatalysts; peptide chemistry; photoredox catalysis.
Figures















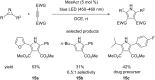

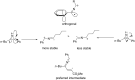




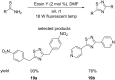


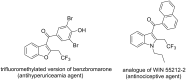












Similar articles
-
Merging Visible Light Photoredox and Gold Catalysis.Acc Chem Res. 2016 Oct 18;49(10):2261-2272. doi: 10.1021/acs.accounts.6b00351. Epub 2016 Sep 9. Acc Chem Res. 2016. PMID: 27610939
-
Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer.Acc Chem Res. 2016 Oct 18;49(10):2295-2306. doi: 10.1021/acs.accounts.6b00270. Epub 2016 Aug 16. Acc Chem Res. 2016. PMID: 27529484 Free PMC article.
-
When Light Meets Nitrogen-Centered Radicals: From Reagents to Catalysts.Acc Chem Res. 2020 May 19;53(5):1066-1083. doi: 10.1021/acs.accounts.0c00090. Epub 2020 Apr 14. Acc Chem Res. 2020. PMID: 32286794
-
Pioneering Metal-Free Late-Stage C-H Functionalization Using Acridinium Salt Photocatalysis.Chemistry. 2024 Dec 2;30(67):e202402809. doi: 10.1002/chem.202402809. Epub 2024 Oct 21. Chemistry. 2024. PMID: 39136621 Review.
-
Progress in Difluoroalkylation of Organic Substrates by Visible Light Photoredox Catalysis.Adv Synth Catal. 2019 Apr 1;361(7):1500-1537. doi: 10.1002/adsc.201801121. Epub 2019 Jan 17. Adv Synth Catal. 2019. PMID: 31680791 Free PMC article. Review.
Cited by
-
Allosteric GABAA Receptor Modulators-A Review on the Most Recent Heterocyclic Chemotypes and Their Synthetic Accessibility.Molecules. 2020 Feb 24;25(4):999. doi: 10.3390/molecules25040999. Molecules. 2020. PMID: 32102309 Free PMC article. Review.
-
PPh3/NaI driven photocatalytic decarboxylative radical cascade alkylarylation reaction of 2-isocyanobiaryls.RSC Adv. 2020 Apr 25;10(28):16510-16514. doi: 10.1039/d0ra03211e. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35498830 Free PMC article.
-
Hydrosulfonylation of Alkenes with Sulfonyl Chlorides under Visible Light Activation.Angew Chem Int Ed Engl. 2020 Jul 6;59(28):11620-11626. doi: 10.1002/anie.202004070. Epub 2020 May 7. Angew Chem Int Ed Engl. 2020. PMID: 32286720 Free PMC article.
-
Visible-Light Photocatalysis as an Enabling Technology for Drug Discovery: A Paradigm Shift for Chemical Reactivity.ACS Med Chem Lett. 2020 Sep 21;11(11):2120-2130. doi: 10.1021/acsmedchemlett.0c00436. eCollection 2020 Nov 12. ACS Med Chem Lett. 2020. PMID: 33214820 Free PMC article.
-
Recent Advances on Metal-Free, Visible-Light- Induced Catalysis for Assembling Nitrogen- and Oxygen-Based Heterocyclic Scaffolds.Molecules. 2019 Apr 18;24(8):1533. doi: 10.3390/molecules24081533. Molecules. 2019. PMID: 31003464 Free PMC article. Review.
References
-
- Reckenthäler M, Griesbeck A G. Adv Synth Catal. 2013;355:2727–2744. doi: 10.1002/adsc.201300751. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
