Revisiting ring-degenerate rearrangements of 1-substituted-4-imino-1,2,3-triazoles
- PMID: 30202463
- PMCID: PMC6122373
- DOI: 10.3762/bjoc.14.184
Revisiting ring-degenerate rearrangements of 1-substituted-4-imino-1,2,3-triazoles
Abstract
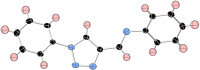
The 1-substituted-4-imino-1,2,3-triazole motif is an established component of coordination compounds and bioactive molecules, but depending on the substituent identity, it can be inherently unstable due to Dimroth rearrangements. This study examined parameters governing the ring-degenerate rearrangement reactions of 1-substituted-4-imino-1,2,3-triazoles, expanding on trends first observed by L'abbé et al. The efficiency of condensation between 4-formyltriazole and amine reactants as well as the propensity of imine products towards rearrangement was each strongly influenced by the substituent identity. It was observed that unsymmetrical condensation reactions conducted at 70 °C produced up to four imine products via a dynamic equilibrium of condensation, rearrangement and hydrolysis steps. Kinetic studies utilizing 1-(4-nitrophenyl)-1H-1,2,3-triazole-4-carbaldehyde with varying amines showed rearrangement rates sensitive to both steric and electronic factors. Such measurements were facilitated by a high throughput colorimetric assay to directly monitor the generation of a 4-nitroaniline byproduct.
Keywords: 1,2,3-triazole; colorimetric assay; condensation; imine exchange; rearrangement.
Figures
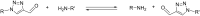


References
-
- Crowley J D, McMorran D A. Top Heterocycl Chem. 2012;28:31–83. doi: 10.1007/7081_2011_67. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
