Tetrathiafulvalene - a redox-switchable building block to control motion in mechanically interlocked molecules
- PMID: 30202469
- PMCID: PMC6122308
- DOI: 10.3762/bjoc.14.190
Tetrathiafulvalene - a redox-switchable building block to control motion in mechanically interlocked molecules
Abstract
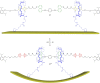
With the rise of artificial molecular machines, control of motion on the nanoscale has become a major contemporary research challenge. Tetrathiafulvalenes (TTFs) are one of the most versatile and widely used molecular redox switches to generate and control molecular motion. TTF can easily be implemented as functional unit into molecular and supramolecular structures and can be reversibly oxidized to a stable radical cation or dication. For over 20 years, TTFs have been key building blocks for the construction of redox-switchable mechanically interlocked molecules (MIMs) and their electrochemical operation has been thoroughly investigated. In this review, we provide an introduction into the field of TTF-based MIMs and their applications. A brief historical overview and a selection of important examples from the past until now are given. Furthermore, we will highlight our latest research on TTF-based rotaxanes.
Keywords: artificial molecular machines; mechanically interlocked molecules; molecular switches; supramolecular chemistry; tetrathiafulvalene.
Figures


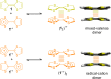
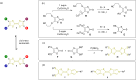
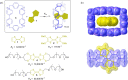
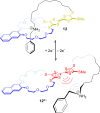
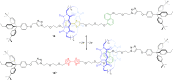
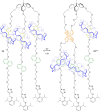
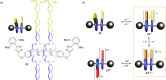
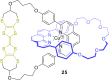
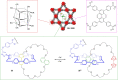
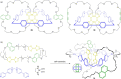
 3 [33]. Solvent molecules and counterions are omitted for clarity.
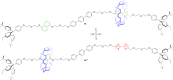
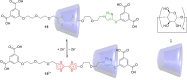
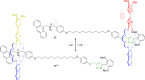
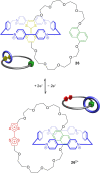
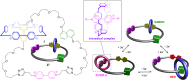
3 [33]. Solvent molecules and counterions are omitted for clarity.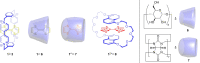

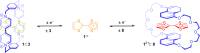






References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
