Abundance of Cytochromes in Hepatic Extracellular Vesicles Is Altered by Drugs Related With Drug-Induced Liver Injury
- PMID: 30202821
- PMCID: PMC6128234
- DOI: 10.1002/hep4.1210
Abundance of Cytochromes in Hepatic Extracellular Vesicles Is Altered by Drugs Related With Drug-Induced Liver Injury
Abstract
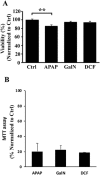
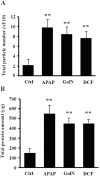
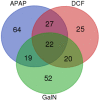
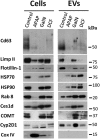
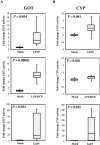
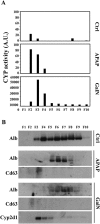
Drug-induced liver injury (DILI) is a serious worldwide health problem that accounts for more than 50% of acute liver failure. There is a great interest in clinical diagnosis and pharmaceutical industry to elucidate underlying molecular mechanisms and find noninvasive biomarkers for this pathology. Cell-secreted extracellular vesicles (EVs) have provided a new biological source to identify low disease invasive markers. Despite the intense research developed on these vesicles, there is currently a gap on their patho-physiological effects. Here, we study EVs secreted by primary rat hepatocytes challenged with galactatosamine (GalN), acetaminophen, or diclofenac as DILI in vitromodels. Proteomics analysis of these EVs revealed an increase in enzymes already associated with liver damage, such as catecholamine-methyl transferase and arginase 1. An increase in translation-related proteins and a decrease in regulators of apoptosis were also observed. In addition, we show the presence of enzymatic activity of P450 cytochrome 2d1 in EVs. The activity specifically is decreased in EVs secreted by hepatocytes after acetaminophen treatment and increased in EVs derived from GalN-treated hepatocytes. By using in vivo preclinical models, we demonstrate the presence of this cytochrome activity in circulation under normal conditions and an increased activity after GalN-induced injury. Conclusion: Hepatocyte-secreted EVs carry active xenobiotic-metabolizing enzymes that might be relevant in extracellular metabolism of drugs and be associated with DILI. (Hepatology Communications 2018;0:00-00).
Figures




References
-
- Sturgill MG, Lambert GH. Xenobiotic‐induced hepatotoxicity: mechanisms of liver injury and methods of monitoring hepatic function. Clin Chem 1997;43:1512‐1526. - PubMed
-
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947‐954. - PubMed
-
- Larrey D. Drug‐induced liver diseases. J Hepatol 2000;32:77‐88. - PubMed
-
- Gunawan B, Kaplowitz N. Clinical perspectives on xenobiotic‐induced hepatotoxicity. Drug Metab Rev 2004;36:301‐312. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

