Oral Versus Aerosolized Ribavirin for the Treatment of Respiratory Syncytial Virus Infections in Hematopoietic Cell Transplant Recipients
- PMID: 30202920
- PMCID: PMC7108094
- DOI: 10.1093/cid/ciy760
Oral Versus Aerosolized Ribavirin for the Treatment of Respiratory Syncytial Virus Infections in Hematopoietic Cell Transplant Recipients
Abstract
Background: The use of oral ribavirin (RBV) for respiratory syncytial virus (RSV) infections is not well studied. With the drastic increase in the cost of aerosolized RBV, we aimed to compare outcomes of hematopoietic cell transplant (HCT) recipients treated with oral or aerosolized RBV for RSV infections.
Methods: We reviewed the records of 124 HCT recipients with RSV infections treated with oral or aerosolized RBV from September 2014 through April 2017. An immunodeficiency scoring index (ISI) was used to classify patients as low, moderate, or high risk for progression to lower respiratory infection (LRI) or death.
Results: Seventy patients (56%) received aerosolized RBV and 54 (44%) oral RBV. Both groups had a 27% rate of progression to LRI (P = 1.00). Mortality rates did not significantly differ between groups (30-day: aerosolized 10%, oral 9%, P = 1.00; 90-day: aerosolized 23%, oral 11%, P = .10). Classification and regression tree analysis identified ISI ≥7 as an independent predictor of 30-day mortality. For patients with ISI ≥7, 30-day mortality was significantly increased overall, yet remained similar between the aerosolized and oral therapy groups (33% for both). After propensity score adjustment, Cox proportional hazards models showed similar mortality rates between oral and aerosolized therapy groups (30-day: hazard ratio [HR], 1.12 [95% confidence interval {CI}, .345-3.65, P = .845).
Conclusions: HCT recipients with RSV infections had similar outcomes when treated with aerosolized or oral RBV. Oral ribavirin may be an effective alternative to aerosolized RBV, with potential significant cost savings.
Keywords: aerosolized ribavirin; hematopoietic cell transplant; oral ribavirin; outcome; respiratory syncytial virus.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
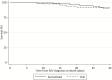
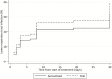
Comment in
-
Reply to Jain et al.Clin Infect Dis. 2019 Nov 27;69(12):2235-2236. doi: 10.1093/cid/ciz379. Clin Infect Dis. 2019. PMID: 31074774 No abstract available.
-
Is Oral Ribavirin for the Treatment of Respiratory Syncytial Virus in High-risk Hematopoietic Stem Cell Transplant Recipients Really Safe?Clin Infect Dis. 2019 Nov 27;69(12):2234-2235. doi: 10.1093/cid/ciz376. Clin Infect Dis. 2019. PMID: 31075174 Free PMC article. No abstract available.
-
Repurposed Oral Ribavirin for Respiratory Virus Infections Requires Pharmacokinetic-pharmacodynamic Dose Optimization.Clin Infect Dis. 2020 Mar 3;70(6):1258. doi: 10.1093/cid/ciz593. Clin Infect Dis. 2020. PMID: 31271195 No abstract available.
References
-
- Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011; 117:2755–63. - PubMed
-
- Khanna N, Widmer AF, Decker M, et al. . Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis 2008; 46:402–12. - PubMed
-
- Martino R, Porras RP, Rabella N, et al. . Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant 2005; 11:781–96. - PMC - PubMed
-
- Raboni SM, Nogueira MB, Tsuchiya LR, et al. . Respiratory tract viral infections in bone marrow transplant patients. Transplantation 2003; 76:142–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

