Development and validation of a prognostic model for overall survival in chemotherapy-naïve men with metastatic castration-resistant prostate cancer
- PMID: 30202945
- PMCID: PMC6888025
- DOI: 10.1093/annonc/mdy406
Development and validation of a prognostic model for overall survival in chemotherapy-naïve men with metastatic castration-resistant prostate cancer
Abstract
Background: Prognostic models are needed that reflect contemporary practice for men with metastatic castration-resistant prostate cancer (mCRPC). We sought to identify predictive and prognostic variables for overall survival (OS) in chemotherapy-naïve men with mCRPC treated with enzalutamide.
Patients and methods: Patients from the PREVAIL trial database (enzalutamide versus placebo) were randomly split 2 : 1 into training (n = 1159) and testing (n = 550) sets. Using the training set, 23 predefined variables were analyzed and a multivariable model predicting OS was developed and validated in an independent testing set.
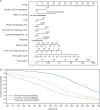
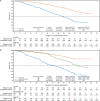
Results: Patient characteristics and outcomes were well balanced between training and testing sets; median OS was 32.7 months in each. The final validated multivariable model included 11 independent prognostic variables. Median OS for low-, intermediate-, and high-risk groups (testing set) defined by prognostic risk tertiles were not yet reached (NYR) (95% CI NYR-NYR), 34.2 months (31.5-NYR), and 21.1 months (17.5-25.0), respectively. Hazard ratios (95% CI) for OS in the low- and intermediate-risk groups versus high-risk group were 0.20 (0.14-0.29) and 0.40 (0.30-0.53), respectively. Secondary outcomes of response and progression differed widely in model-defined risk groups. Enzalutamide improved outcomes in all prognostic risk groups.
Conclusions: Our validated prognostic model incorporates variables routinely collected in chemotherapy-naïve men with mCRPC treated with enzalutamide, identifying subsets of patients with widely differing survival outcomes that provide useful information for external validation, patient care, and clinical trial design.
Trial registration: ClinicalTrials.gov: NCT01212991.
Figures
Comment in
-
Developing prognostic models for advanced prostate cancer when the goal line keeps changing.Ann Oncol. 2018 Nov 1;29(11):2155-2157. doi: 10.1093/annonc/mdy419. Ann Oncol. 2018. PMID: 30307525 No abstract available.
References
-
- Ferlay J, Soerjomataram I, Ervik M. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11, 2016. http://globocan.iarc.fr (31 May 2018, date last accessed).
-
- Scher HI, Fizazi K, Saad F. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367(13): 1187–1197. - PubMed
-
- Fizazi K, Scher HI, Molina A. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13(10): 983–992. - PubMed
-
- Kantoff PW, Higano CS, Shore ND. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363(5): 411–422. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

