The Enabling Reduction of Low-Grade Inflammation in Seniors (ENRGISE) Pilot Study: Screening Methods and Recruitment Results
- PMID: 30202946
- PMCID: PMC6625594
- DOI: 10.1093/gerona/gly204
The Enabling Reduction of Low-Grade Inflammation in Seniors (ENRGISE) Pilot Study: Screening Methods and Recruitment Results
Abstract
Background: The Enabling Reduction of Low-grade Inflammation in Seniors (ENRGISE) Pilot Study is a multicenter randomized clinical trial examining the feasibility of testing whether omega-3 fish oil (ω-3) and the angiotensin receptor blocker losartan alone or in combination can reduce inflammation and improve walking speed in older adults with mobility impairment. We describe recruitment methods and results.
Methods: Eligible participants were 70 years and older, had elevated interleukin-6 levels (2.5-30 pg/mL) and mobility impairment.
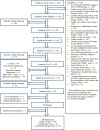
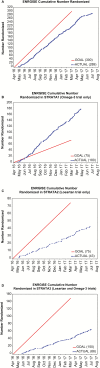
Results: Of those who responded to recruitment, 83% responded to mailings. A total of 5,424 telephone screens were completed; of these, 2,011 (37.1%) were eligible for further screening. The most common reasons for ineligibility at the telephone screens were lack of mobility impairment or use of angiotensin receptor blockers or angiotensin-converting enzyme inhibitors (n=1.789). Of the 1,305 initial screening visits, 1,087 participants had slow gait speed (<1 m/s). Of these, 701 (64%) had elevated interleukin-6 and were eligible for second screening visits. Of the 582 second screening visits, 335 (57.6%) were eligible to be randomized. A total of 289 participants (96% of goal) were randomized: 180 in the ω-3 stratum (240% of goal); 43 in the losartan (57% of goal), and 66 in the combination (44% of goal). The telephone screen and first screening visit to randomization ratio was 19 to 1 and 4.5 to 1, respectively. The estimated cost of recruitment per randomized participant was $1,782.
Conclusion: Recruitment for ω-3 exceeded goals, but goals for the losartan and combination strata were not met due to the high proportion of participants taking angiotensin receptor blockers or angiotensin-converting enzyme inhibitors.
Keywords: Clinical trials; Functional performance; Inflammation; Mobility impairment; Physical function.
© The Author(s) 2018. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Anton S, Sourdet S, Pahor M, Manini TM. Challenges in implementing large-scale clinical trials in moderately functioning older adults. In: Cherubini A, Bernabei R, Ferrucci L, Marchionni N, Studenski S, Vellas B, eds. Clinical Trials in Older Adults. Hoboken, NJ: Wiley Blackwell; 2015:125–152.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

