Role of plasmid plasticity and mobile genetic elements in the entomopathogen Bacillus thuringiensis serovar israelensis
- PMID: 30203090
- PMCID: PMC6199540
- DOI: 10.1093/femsre/fuy034
Role of plasmid plasticity and mobile genetic elements in the entomopathogen Bacillus thuringiensis serovar israelensis
Abstract
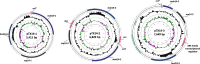
Bacillus thuringiensis is a well-known biopesticide that has been used for more than 80 years. This spore-forming bacterium belongs to the group of Bacillus cereus that also includes, among others, emetic and diarrheic pathotypes of B. cereus, the animal pathogen Bacillus anthracis and the psychrotolerant Bacillus weihenstephanensis. Bacillus thuringiensis is rather unique since it has adapted its lifestyle as an efficient pathogen of specific insect larvae. One of the peculiarities of B. thuringiensis strains is the extent of their extrachromosomal pool, with strains harbouring more than 10 distinct plasmid molecules. Among the numerous serovars of B. thuringiensis, 'israelensis' is certainly emblematic since its host spectrum is apparently restricted to dipteran insects like mosquitoes and black flies, vectors of human and animal diseases such as malaria, yellow fever, or river blindness. In this review, the putative role of the mobile gene pool of B. thuringiensis serovar israelensis in its pathogenicity and dedicated lifestyle is reviewed, with specific emphasis on the nature, diversity, and potential mobility of its constituents. Variations among the few related strains of B. thuringiensis serovar israelensis will also be reported and discussed in the scope of this specialised insect pathogen, whose lifestyle in the environment remains largely unknown.
Figures






References
-
- Adriaenssens EM, Wittmann J, Kuhn JH et al. Taxonomy of prokaryotic viruses: 2017 update from the ICTV Bacterial and archaeal viruses subcommittee. Arch Virol 2018;163:1125–9. - PubMed
-
- Ackermann HW, Roy R, Martin M et al. Partial characterization of a cubic Bacillus phage. Can J Microbiol 1978;24:986–93. - PubMed
-
- Ahn C, Collins-Thompson D, Duncan C et al. Mobilization and location of the genetic determinant of chloramphenicol resistance from Lactobacillus plantarum caTC2R. Plasmid 1992;27:169–76. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

