Brown Adipose Tissue Development and Metabolism
- PMID: 30203328
- PMCID: PMC7330484
- DOI: 10.1007/164_2018_168
Brown Adipose Tissue Development and Metabolism
Abstract
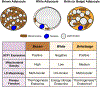
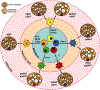
Brown adipose tissue is well known to be a thermoregulatory organ particularly important in small rodents and human infants, but it was only recently that its existence and significance to metabolic fitness in adult humans have been widely realized. The ability of active brown fat to expend high amounts of energy has raised interest in stimulating thermogenesis therapeutically to treat metabolic diseases related to obesity and type 2 diabetes. In parallel, there has been a surge of research aimed at understanding the biology of rodent and human brown fat development, its remarkable metabolic properties, and the phenomenon of white fat browning, in which white adipocytes can be converted into brown like adipocytes with similar thermogenic properties. Here, we review the current understanding of the developmental and metabolic pathways involved in forming thermogenic adipocytes, and highlight some of the many unknown functions of brown fat that make its study a rich and exciting area for future research.
Keywords: Adipogenesis; Beige adipocyte; Brite adipocyte; Brown adipose tissue; Development; Glucose and lipid metabolism; Lineage tracing; Progenitor cells; Thermogenesis; Ucp1.
Figures

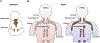
References
-
- AHFELDT T, SCHINZEL RT, LEE Y-K, HENDRICKSON D, KAPLAN A, LUM DH, CAMAHORT R, XIA F, SHAY J, RHEE EP, CLISH CB, DEO RC, SHEN T, LAU FH, COWLEY A, MOWRER G, AL-SIDDIQI H, NAHRENDORF M, MUSUNURU K, GERSZTEN RE, RINN JL & COWAN CA 2012. Programming human pluripotent stem cells into white and brown adipocytes. Nature Cell Biology, 14, 209–219. - PMC - PubMed
-
- AMBROSI TH, SCIALDONE A, GRAJA A, GOHLKE S, JANK AM, BOCIAN C, WOELK L, FAN H, LOGAN DW, SCHURMANN A, SARAIVA LR & SCHULZ TJ 2017. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell, 20, 771–784.e6. - PMC - PubMed
-
- ATIT R, SGAIER SK, MOHAMED OA, TAKETO MM, DUFORT D, JOYNER AL, NISWANDER L & CONLON RA 2006. beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental Biology, 296, 164–176. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

