A Functional Tissue-Engineered Synovium Model to Study Osteoarthritis Progression and Treatment
- PMID: 30203722
- PMCID: PMC6482911
- DOI: 10.1089/ten.TEA.2018.0142
A Functional Tissue-Engineered Synovium Model to Study Osteoarthritis Progression and Treatment
Abstract
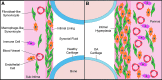
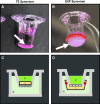
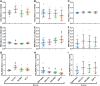
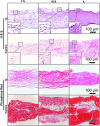
The synovium envelops the diarthrodial joint and plays a key regulatory role in defining the composition of the synovial fluid through filtration and biosynthesis of critical boundary lubricants. Synovium changes often precede cartilage damage in osteoarthritis. We describe a novel in vitro tissue engineered model, validated against native synovium explants, to investigate the structure-function of synovium through quantitative solute transport measures. Synovium was evaluated in the presence of a proinflammatory cytokine, interleukin-1, or the clinically relevant corticosteroid, dexamethasone. We anticipate that a better understanding of synovium transport would support efforts to develop more effective strategies aimed at restoring joint health.
Keywords: models; osteoarthritis; solute transport; synovium.
Conflict of interest statement
No competing financial interests exist.
Figures



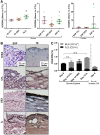
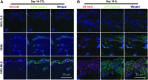
References
-
- Schmidt T.A., and Sah R.L. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage 15, 35, 2007 - PubMed
-
- Kiener H.P., Watts G.F.M., Cui Y., et al. . Synovial fibroblasts self-direct multicellular lining architecture and synthetic function in three-dimensional organ culture. Arthritis Rheum 62, 742, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

