Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates
- PMID: 30204075
- PMCID: PMC6209069
- DOI: 10.1148/radiol.2018181151
Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates
Abstract
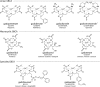
Gadolinium-based contrast agents (GBCAs) have revolutionized MRI, enabling physicians to obtain crucial life-saving medical information that often cannot be obtained with other imaging modalities. Since initial approval in 1988, over 450 million intravenous GBCA doses have been administered worldwide, with an extremely favorable pharmacologic safety profile; however, recent information has raised new concerns over the safety of GBCAs. Mounting evidence has shown there is long-term retention of gadolinium in human tissues. Further, a small subset of patients have attributed a constellation of symptoms to GBCA exposure, although the association of these symptoms with GBCA administration or gadolinium retention has not been proven by scientific investigation. Despite evidence that macrocyclic GBCAs show less gadolinium retention than linear GBCAs, the safety implications of gadolinium retention are unknown. The mechanism and chemical forms of gadolinium retention, as well as the biologic activity and clinical importance of these retained gadolinium species, remain poorly understood and underscore the need for additional research. In February 2018, an international meeting was held in Bethesda, Md, at the National Institutes of Health to discuss the current literature and knowledge gaps about gadolinium retention, to prioritize future research initiatives to better understand this phenomenon, and to foster collaborative standardized studies. The greatest priorities are to determine (a) if gadolinium retention adversely affects the function of human tissues, (b) if retention is causally associated with short- or long-term clinical manifestations of disease, and (c) if vulnerable populations, such as children, are at greater risk for experiencing clinical disease. The purpose of the research roadmap is to highlight important information that is not known and to identify and prioritize needed research. ©RSNA, 2018 Online supplemental material is available for this article .
Figures
References
-
- Balzer T. Presence of gadolinium (Gd) in the brain and body. Presentation to the Medical Imaging Drugs Advisory Committee, FDA. Silver Spring, Md: U.S. Food and Drug Administration, 2017.
-
- Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 2011;196(2):W138–W143. - PubMed
-
- Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate allergic reactions to gadolinium-based contrast agents: a systematic review and meta-analysis. Radiology 2018;286(2):471–482. - PubMed
-
- Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol 2009;193(4):1124–1127. - PubMed
-
- Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007;56(1):27–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

