Identification of cryptic subunits from an apicomplexan ATP synthase
- PMID: 30204085
- PMCID: PMC6133553
- DOI: 10.7554/eLife.38097
Identification of cryptic subunits from an apicomplexan ATP synthase
Abstract
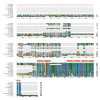
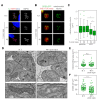
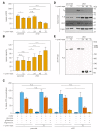
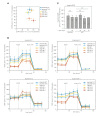
The mitochondrial ATP synthase is a macromolecular motor that uses the proton gradient to generate ATP. Proper ATP synthase function requires a stator linking the catalytic and rotary portions of the complex. However, sequence-based searches fail to identify genes encoding stator subunits in apicomplexan parasites like Toxoplasma gondii or the related organisms that cause malaria. Here, we identify 11 previously unknown subunits from the Toxoplasma ATP synthase, which lack homologs outside the phylum. Modeling suggests that two of them, ICAP2 and ICAP18, are distantly related to mammalian stator subunits. Our analysis shows that both proteins form part of the ATP synthase complex. Depletion of ICAP2 leads to aberrant mitochondrial morphology, decreased oxygen consumption, and disassembly of the complex, consistent with its role as an essential component of the Toxoplasma ATP synthase. Our findings highlight divergent features of the central metabolic machinery in apicomplexans, which may reveal new therapeutic opportunities.
Keywords: ATP synthase; Apicomplexa; Toxoplasma gondii; cell biology; infectious disease; microbiology; mitochondria.
© 2018, Huet et al.
Conflict of interest statement
DH, ER, Gv, SL No competing interests declared
Figures







References
-
- Balabaskaran Nina P, Morrisey JM, Ganesan SM, Ke H, Pershing AM, Mather MW, Vaidya AB. ATP synthase complex of plasmodium falciparum: dimeric assembly in mitochondrial membranes and resistance to genetic disruption. The Journal of Biological Chemistry. 2011;286:41312–41322. doi: 10.1074/jbc.M111.290973. - DOI - PMC - PubMed
-
- Baracca A, Sgarbi G, Mattiazzi M, Casalena G, Pagnotta E, Valentino ML, Moggio M, Lenaz G, Carelli V, Solaini G. Biochemical phenotypes associated with the mitochondrial ATP6 gene mutations at nt8993. Biochimica Et Biophysica Acta (BBA) - Bioenergetics. 2007;1767:913–919. doi: 10.1016/j.bbabio.2007.05.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

