PERK regulates skeletal muscle mass and contractile function in adult mice
- PMID: 30204503
- PMCID: PMC6338633
- DOI: 10.1096/fj.201800683RR
PERK regulates skeletal muscle mass and contractile function in adult mice
Abstract
Skeletal muscle mass is regulated by the coordinated activation of several anabolic and catabolic pathways. The endoplasmic reticulum (ER) is a major site of protein folding and a reservoir for calcium ions. Accretion of misfolded proteins or depletion in calcium concentration causes stress in the ER, which leads to the activation of a signaling network known as the unfolded protein response (UPR). In the present study, we investigated the role of the protein kinase R-like endoplasmic reticulum kinase (PERK) arm of the UPR in the regulation of skeletal muscle mass and function in naive conditions and in a mouse model of cancer cachexia. Our results demonstrate that the targeted inducible deletion of PERK reduces skeletal muscle mass, strength, and force production during isometric contractions. Deletion of PERK also causes a slow-to-fast fiber type transition in skeletal muscle. Furthermore, short hairpin RNA-mediated knockdown or pharmacologic inhibition of PERK leads to atrophy in cultured myotubes. While increasing the rate of protein synthesis, the targeted deletion of PERK leads to the increased expression of components of the ubiquitin-proteasome system and autophagy in skeletal muscle. Ablation of PERK also increases the activation of calpains and deregulates the gene expression of the members of the FGF19 subfamily. Furthermore, the targeted deletion of PERK increases muscle wasting in Lewis lung carcinoma tumor-bearing mice. Our findings suggest that the PERK arm of the UPR is essential for the maintenance of skeletal muscle mass and function in adult mice.-Gallot, Y. S., Bohnert, K. R., Straughn, A. R., Xiong, G., Hindi, S. M., Kumar, A. PERK regulates skeletal muscle mass and contractile function in adult mice.
Keywords: ER stress; cancer cachexia; lewis lung carcinoma; proteostasis; unfolded protein response.
Conflict of interest statement
This work was supported by U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Disease Grants AG029623, AR059810 and AR068313 (to A.K.) Figure 1
Figures


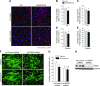
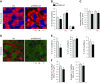
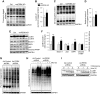
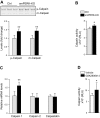
Similar articles
-
Inhibition of ER stress and unfolding protein response pathways causes skeletal muscle wasting during cancer cachexia.FASEB J. 2016 Sep;30(9):3053-68. doi: 10.1096/fj.201600250RR. Epub 2016 May 20. FASEB J. 2016. PMID: 27206451 Free PMC article.
-
A Systems Biological View of Life-and-Death Decision with Respect to Endoplasmic Reticulum Stress-The Role of PERK Pathway.Int J Mol Sci. 2017 Jan 5;18(1):58. doi: 10.3390/ijms18010058. Int J Mol Sci. 2017. PMID: 28067773 Free PMC article.
-
The PERK arm of the unfolded protein response regulates satellite cell-mediated skeletal muscle regeneration.Elife. 2017 Mar 23;6:e22871. doi: 10.7554/eLife.22871. Elife. 2017. PMID: 28332979 Free PMC article.
-
Hidden Agenda - The Involvement of Endoplasmic Reticulum Stress and Unfolded Protein Response in Inflammation-Induced Muscle Wasting.Front Immunol. 2022 May 9;13:878755. doi: 10.3389/fimmu.2022.878755. eCollection 2022. Front Immunol. 2022. PMID: 35615361 Free PMC article. Review.
-
Confounding Roles of ER Stress and the Unfolded Protein Response in Skeletal Muscle Atrophy.Int J Mol Sci. 2021 Mar 4;22(5):2567. doi: 10.3390/ijms22052567. Int J Mol Sci. 2021. PMID: 33806433 Free PMC article. Review.
Cited by
-
Emerging role of TAK1 in the regulation of skeletal muscle mass.Bioessays. 2023 Apr;45(4):e2300003. doi: 10.1002/bies.202300003. Epub 2023 Feb 15. Bioessays. 2023. PMID: 36789559 Free PMC article. Review.
-
The Toll-Like Receptor/MyD88/XBP1 Signaling Axis Mediates Skeletal Muscle Wasting during Cancer Cachexia.Mol Cell Biol. 2019 Jul 16;39(15):e00184-19. doi: 10.1128/MCB.00184-19. Print 2019 Aug 1. Mol Cell Biol. 2019. PMID: 31138662 Free PMC article.
-
Mechanisms, assessment, and exercise interventions for skeletal muscle dysfunction post-chemotherapy in breast cancer: from inflammation factors to clinical practice.Front Oncol. 2025 Mar 4;15:1551561. doi: 10.3389/fonc.2025.1551561. eCollection 2025. Front Oncol. 2025. PMID: 40104495 Free PMC article. Review.
-
Protective effects of Salubrinal against H2O2-induced muscle wasting via eIF2α/ATF4 signaling pathway.Front Pharmacol. 2025 Jun 27;16:1607606. doi: 10.3389/fphar.2025.1607606. eCollection 2025. Front Pharmacol. 2025. PMID: 40657639 Free PMC article.
-
Mechanisms of muscle atrophy and hypertrophy: implications in health and disease.Nat Commun. 2021 Jan 12;12(1):330. doi: 10.1038/s41467-020-20123-1. Nat Commun. 2021. PMID: 33436614 Free PMC article. Review.
References
-
- Kandarian, S. C., Jackman, R. W. (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33, 155–165 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

