Elobixibat for the treatment of constipation
- PMID: 30204504
- PMCID: PMC6386599
- DOI: 10.1080/17474124.2018.1522248
Elobixibat for the treatment of constipation
Abstract
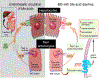
Chronic idiopathic constipation (CC) is highly prevalent worldwide. A subset of patients with CC have reduced fecal (and by inference, intra-colonic) bile acids (BA). Elobixibat, a locally-acting ileal bile acid transporter (IBAT) inhibitor, leads to increased BA delivery to the colon and represents a new class of treatment for CC. BAs accelerate colonic transit and increase colonic secretion. Therefore, IBAT inhibitors have potential to treat patients with CC. Areas covered: Rationale for IBAT inhibitor in therapeutics, and preclinical and clinical pharmacology of elobixibat: In vitro, elobixibat is a highly potent, selective IBAT inhibitor. In humans, elobixibat accelerated colonic transit. In phase 2A, 2B and 3 studies in CC, elobixibat was efficacious, well tolerated and safe. An open-label, phase 3 trial (52 weeks) confirmed the safety of elobixibat. Elobixibat reduces LDL cholesterol, increases serum GLP-1, and has potential in metabolic syndrome. Expert commentary: Uniquely among current treatments of CC, elobixibat stimulates both motor and secretory functions in the colon. These dual effects suggest that, when approved, elobixibat may be a first-line choice for constipation associated with colonic BA deficiency and a second-line treatment for all patients with CC and constipation-predominant irritable bowel syndrome. Further studies are required to confirm efficacy for relief of CC. Once approved, elobixibat will likely become a second-line choice for treatment of CC.
Keywords: Bile acid; enterohepatic circulation; ileal bile acid transporter (IBAT); irritable bowel syndrome; pharmacodynamics; pharmacokinetics.
Figures


Similar articles
-
Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial.Lancet Gastroenterol Hepatol. 2018 Aug;3(8):537-547. doi: 10.1016/S2468-1253(18)30123-7. Epub 2018 May 25. Lancet Gastroenterol Hepatol. 2018. PMID: 29805116 Clinical Trial.
-
Elobixibat, the first-in-class Ileal Bile Acid Transporter inhibitor, for the treatment of Chronic Idiopathic Constipation.Expert Opin Pharmacother. 2018 Aug;19(12):1381-1388. doi: 10.1080/14656566.2018.1508450. Epub 2018 Aug 21. Expert Opin Pharmacother. 2018. PMID: 30129377 Review.
-
Elobixibat for the treatment of constipation.Expert Opin Investig Drugs. 2013 Feb;22(2):277-84. doi: 10.1517/13543784.2013.753056. Epub 2012 Dec 8. Expert Opin Investig Drugs. 2013. PMID: 23215781 Review.
-
Determining an optimal clinical dose of elobixibat, a novel inhibitor of the ileal bile acid transporter, in Japanese patients with chronic constipation: a phase II, multicenter, double-blind, placebo-controlled randomized clinical trial.J Gastroenterol. 2018 Apr;53(4):525-534. doi: 10.1007/s00535-017-1383-5. Epub 2017 Aug 24. J Gastroenterol. 2018. PMID: 28840422 Free PMC article. Clinical Trial.
-
Specific inhibition of bile acid transport alters plasma lipids and GLP-1.BMC Cardiovasc Disord. 2015 Jul 22;15:75. doi: 10.1186/s12872-015-0070-9. BMC Cardiovasc Disord. 2015. PMID: 26197999 Free PMC article. Clinical Trial.
Cited by
-
The Effect of Chronic Laxative Use on Lipid Profile and HbA1c: A Hospital-Based Retrospective Study.Cureus. 2023 Sep 11;15(9):e45055. doi: 10.7759/cureus.45055. eCollection 2023 Sep. Cureus. 2023. PMID: 37829969 Free PMC article.
-
Medicinal Formula Huazhi-Rougan Attenuates Non-Alcoholic Steatohepatitis Through Enhancing Fecal Bile Acid Excretion in Mice.Front Pharmacol. 2022 Jun 1;13:833414. doi: 10.3389/fphar.2022.833414. eCollection 2022. Front Pharmacol. 2022. PMID: 35721143 Free PMC article.
-
Deoxycholic acid inducing chronic atrophic gastritis with colonic mucosal lesion correlated to mucosal immune dysfunction in rats.Sci Rep. 2024 Jul 9;14(1):15798. doi: 10.1038/s41598-024-66660-3. Sci Rep. 2024. PMID: 38982226 Free PMC article.
-
Efficacy and safety of elobixibat in combination with or switched from conventional treatments of chronic constipation: A retrospective observational study.JGH Open. 2024 Aug 26;8(8):e70019. doi: 10.1002/jgh3.70019. eCollection 2024 Aug. JGH Open. 2024. PMID: 39193138 Free PMC article.
-
Effects of Elobixibat on Constipation and Lipid Metabolism in Patients With Moderate to End-Stage Chronic Kidney Disease.Front Med (Lausanne). 2022 Jan 17;8:780127. doi: 10.3389/fmed.2021.780127. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35111776 Free PMC article.
References
-
- Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, Rhoten D, Lueke A, Saenger A, Girtman A, Zinsmeister AR: Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol (2013) 11(10):1270–1275 e1271. - PMC - PubMed
-
- Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ: Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by sehcat scanning in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics (2009) 30(7):707–717. - PubMed
-
- Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhea in functional bowel disorder with diarrhea: a systematic review and meta-analysis. Gut (2016) 65(12):1951–1959. - PubMed
-
- Vijayvargiya P, Camilleri M: Update on bile acid malabsorption: Finally ready for prime time? Current Gastroenterology Reports (2018) 20(3):10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
