The serum vitamin D metabolome: What we know and what is still to discover
- PMID: 30205156
- PMCID: PMC6342654
- DOI: 10.1016/j.jsbmb.2018.09.003
The serum vitamin D metabolome: What we know and what is still to discover
Abstract
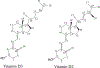
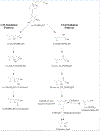
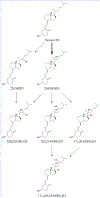
Vitamin D, referring to the two forms, D2 from the diet and D3 primarily derived from phototransformation in the skin, is a prohormone important in human health. The most hormonally active form, 1α,25-dihydroxyvitamin D (1α,25(OH)2D), formed from vitamin D via 25-hydroxyvitamin D (25(OH)D), is not only important for regulating calcium metabolism, but has many pleiotropic effects including regulation of the immune system and has anti-cancer properties. The major circulating form of vitamin D is 25(OH)D and both D2 and D3 forms are routinely measured by LC/MS/MS to assess vitamin D status, due to their relatively long half-lives and much higher concentrations compared to 1α,25(OH)2D. Inactivation of both 25(OH)D and 1α,25(OH)2D is catalyzed by CYP24A1 and 25-hydroxyvitamin D3 3-epimerase. Initial products from these enzymes acting on 25(OH)D3 are 24R,25(OH)2D3 and 3-epi-25(OH)D3, respectively, and both of these can also be measured routinely in some clinical laboratories to further document vitamin D status. With advances in LC/MS/MS and its increased availability, and with the help of studies with recombinant vitamin D-metabolizing enzymes, many other vitamin D metabolites have now been detected and in some cases quantitated, in human serum. CYP11A1 which catalyzes the first step in steroidogenesis, has been found to also act on vitamins D3 and D2 hydroxylating both at C20, but with some secondary metabolites produced by subsequent hydroxylations at other positions on the side chain. The major vitamin D3 metabolite, 20S-hydroxyvitamin D3 (20S(OH)D3), shows biological activity, often similar to 1α,25(OH)2D3 but without calcemic effects. Using standards produced enzymatically by purified CYP11A1 and characterized by NMR, many of these new metabolites have been detected in human serum, with semi-quantitative measurement of 20S(OH)D3 indicating it is present at comparable concentrations to 24R,25(OH)2D3 and 3-epi-25(OH)D3. Recently, vitamin D-related hydroxylumisterols derived from lumisterol3, a previtamin D3 photoproduct, have also been measured in human serum and displayed biological activity in initial in vitro studies. With the current extensive knowledge on the reactions and pathways of metabolism of vitamin D, especially those catalyzed by CYP24A1, CYP27A1, CYP27B1, CYP3A4 and CYP11A1, it is likely that many other of the resulting hydroxyvitamin D metabolites will be measured in human serum in the future, some contributing to a more detailed understanding of vitamin D status in health and disease.
Keywords: 25-Hydroxyvitamin D3; Cytochrome P450; LC/MS/MS; Metabolome; Vitamin D.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures

References
-
- Holick MF, Vitamin D deficiency N Engl J Med, 357 (2007) 266–281. - PubMed
-
- Holick MF, Vitamin D: A millenium perspective, Journal of cellular biochemistry, 88 (2003) 296–307. - PubMed
-
- Zhu J, DeLuca HF, Vitamin D 25-hydroxylase - Four decades of searching, are we there yet?, Archives of biochemistry and biophysics, 523 (2012) 30–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

