Differential regulation of cytokine and chemokine expression by MK2 and MK3 in airway smooth muscle cells
- PMID: 30205157
- PMCID: PMC9036849
- DOI: 10.1016/j.pupt.2018.09.004
Differential regulation of cytokine and chemokine expression by MK2 and MK3 in airway smooth muscle cells
Abstract
Background: Airway smooth muscle (ASM) contributes to local inflammation and plays an immunomodulatory role in airway diseases. This is partially regulated by p38 mitogen-activated protein kinase (MAPK), which further activates two closely related isoforms of the MAPK-activated protein kinases (MKs), MK2 and MK3. The MKs have similar substrate specificities but less is known about differences in their functional responses. This study was undertaken to identify differential downstream inflammatory targets of MK2 and MK3 signaling and assess cross-talk between the MAPK pathway and NF-κB signaling relevant to ASM function.
Methods: Wild-type and kinase-deficient MK2 (MK2WT, MK2KR) and MK3 (MK3WT, MK33A) were expressed in human ASM cells stimulated for 20 h with 10 ng/ml each interleukin (IL)-1β, tumor necrosis factor (TNF)-α and interferon (IFN)-γ. Inflammatory mediator secretion was assessed by Luminex assays and ELISA. Signaling pathway activation was monitored by Western blotting.
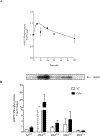
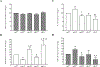
Results: Expression of these MKs and stimulation with 10 ng/ml IL-1β, TNFα and IFNγ for 20 h did not affect secretion of multiple cytokines including IL-4, IL-5, IL-13 and monocyte chemotactic protein (MCP)-1/CCL2 but did differentially affect the secretion of regulated upon activation, normal T cell expressed and secreted (RANTES)/CCL5, IL-6 and granulocyte macrophage-colony stimulating factor (GM-CSF). RANTES/CCL5 secretion was decreased by MK2WT or MK3WT and stimulated by inhibition of MK2 or MK3 activity with expression of the kinase-deficient enzymes MK2KR or MK33A. IL-6 and GM-CSF secretion was decreased by inhibition of MK2 activity with MK2KR and while MK3WT had no effect, the kinase-deficient MK33A further decreased secretion of these mediators. Cross-talk of the MKs with other signaling pathways was investigated by examining NF-κB activation, which was inhibited by expression of MK3 but not affected by MK2.
Conclusions: These results suggest an inhibitory role for MK2 and MK3 activity in RANTES/CCL5 secretion and cross-talk of MK3 with NF-κB to regulate IL-6 and GM-CSF. These findings differentiate MK2 and MK3 function in ASM cells and provide insight that may enable selective targeting of MKs in ASM to modulate local inflammation in airway disease.
Keywords: Airways; Asthma; Cell signaling; Inflammation.
Copyright © 2018. Published by Elsevier Ltd.
Conflict of interest statement
Figures


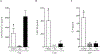
References
-
- Hedges JC, Singer CA, Gerthoffer WT. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol 2000; 23:86–94. - PubMed
-
- Hallsworth MP, Moir LM, Lai D, Hirst SJ. Inhibitors of mitogen-activated protein kinases differentially regulate eosinophil-activating cytokine release from human airway smooth muscle. Am J Respir Crit Care Med 2001; 164:688–697. - PubMed
-
- Gerthoffer WT, Singer CA. MAPK regulation of gene expression in airway smooth muscle. Respir Physiol Neurobiol 2003; 137:237–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

