The Physicochemical Properties of Decellularized Extracellular Matrix-Coated 3D Printed Poly(ε-caprolactone) Nerve Conduits for Promoting Schwann Cells Proliferation and Differentiation
- PMID: 30205596
- PMCID: PMC6164117
- DOI: 10.3390/ma11091665
The Physicochemical Properties of Decellularized Extracellular Matrix-Coated 3D Printed Poly(ε-caprolactone) Nerve Conduits for Promoting Schwann Cells Proliferation and Differentiation
Abstract
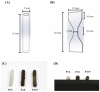
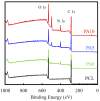
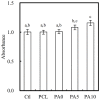
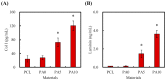
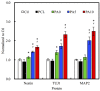
Although autologous nerve grafting remains the gold standard treatment for peripheral nerve injuries, alternative methods such as development of nerve guidance conduits have since emerged and evolved to counter the many disadvantages of nerve grafting. However, the efficacy and viability of current nerve conduits remain unclear in clinical trials. Here, we focused on a novel decellularized extracellular matrix (dECM) and polydopamine (PDA)-coated 3D-printed poly(ε-caprolactone) (PCL)-based conduits, whereby the PDA surface modification acts as an attachment platform for further dECM attachment. We demonstrated that dECM/PDA-coated PCL conduits possessed higher mechanical properties when compared to human or animal nerves. Such modifications were proved to affect cell behaviors. Cellular behaviors and neuronal differentiation of Schwann cells were assessed to determine for the efficacies of the conduits. There were some cell-specific neuronal markers, such as Nestin, neuron-specific class III beta-tubulin (TUJ-1), and microtubule-associated protein 2 (MAP2) analyzed by enzyme-linked immunosorbent assay, and Nestin expressions were found to be 0.65-fold up-regulated, while TUJ1 expressions were 2.3-fold up-regulated and MAP2 expressions were 2.5-fold up-regulated when compared to Ctl. The methodology of PDA coating employed in this study can be used as a simple model to immobilize dECM onto PCL conduits, and the results showed that dECM/PDA-coated PCL conduits can as a practical and clinically viable tool for promoting regenerative outcomes in larger peripheral nerve defects.
Keywords: autologous nerve grafting; decellularized extracellular matrix; neuronal differentiation; poly(ε-caprolactone) conduits; polydopamine.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






Similar articles
-
Promoting regeneration of peripheral nerves in-vivo using new PCL-NGF/Tirofiban nerve conduits.Biomaterials. 2011 Jan;32(3):734-43. doi: 10.1016/j.biomaterials.2010.09.023. Biomaterials. 2011. PMID: 20888633
-
Additive Manufacturing of Nerve Decellularized Extracellular Matrix-Contained Polyurethane Conduits for Peripheral Nerve Regeneration.Polymers (Basel). 2019 Oct 4;11(10):1612. doi: 10.3390/polym11101612. Polymers (Basel). 2019. PMID: 31590259 Free PMC article.
-
Pulmonary decellularized extracellular matrix (dECM) modified polyethylene terephthalate three-dimensional cell carriers regulate the proliferation and paracrine activity of mesenchymal stem cells.Front Bioeng Biotechnol. 2024 Jan 8;11:1324424. doi: 10.3389/fbioe.2023.1324424. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38260733 Free PMC article.
-
Therapeutic strategies for peripheral nerve injury: decellularized nerve conduits and Schwann cell transplantation.Neural Regen Res. 2019 Aug;14(8):1343-1351. doi: 10.4103/1673-5374.253511. Neural Regen Res. 2019. PMID: 30964052 Free PMC article. Review.
-
Decellularized Extracellular Matrix for Tissue Engineering (Review).Sovrem Tekhnologii Med. 2022;14(3):57-68. doi: 10.17691/stm2022.14.3.07. Epub 2022 May 28. Sovrem Tekhnologii Med. 2022. PMID: 37064810 Free PMC article. Review.
Cited by
-
Recent Advances in Decellularized Matrix-Derived Materials for Bioink and 3D Bioprinting.Gels. 2023 Mar 3;9(3):195. doi: 10.3390/gels9030195. Gels. 2023. PMID: 36975644 Free PMC article. Review.
-
Applications of Polydopamine-Modified Scaffolds in the Peripheral Nerve Tissue Engineering.Front Bioeng Biotechnol. 2020 Oct 21;8:590998. doi: 10.3389/fbioe.2020.590998. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195158 Free PMC article. Review.
-
Development and In Vitro Differentiation of Schwann Cells.Cells. 2022 Nov 24;11(23):3753. doi: 10.3390/cells11233753. Cells. 2022. PMID: 36497014 Free PMC article. Review.
-
Improved Bioactivity of 3D Printed Porous Titanium Alloy Scaffold with Chitosan/Magnesium-Calcium Silicate Composite for Orthopaedic Applications.Materials (Basel). 2019 Jan 9;12(2):203. doi: 10.3390/ma12020203. Materials (Basel). 2019. PMID: 30634440 Free PMC article.
-
A critical review on polydopamine surface-modified scaffolds in musculoskeletal regeneration.Front Bioeng Biotechnol. 2022 Nov 18;10:1008360. doi: 10.3389/fbioe.2022.1008360. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36466324 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

