Arabidopsis PIRL6 Is Essential for Male and Female Gametogenesis and Is Regulated by Alternative Splicing
- PMID: 30206104
- PMCID: PMC6236607
- DOI: 10.1104/pp.18.00329
Arabidopsis PIRL6 Is Essential for Male and Female Gametogenesis and Is Regulated by Alternative Splicing
Abstract
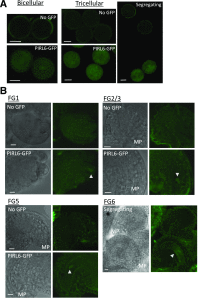
Plant intracellular Ras-group leucine-rich repeat (LRR) proteins (PIRLs) are related to Ras-interacting animal LRR proteins that participate in developmental cell signaling. Systematic knockout analysis has implicated some members of the Arabidopsis (Arabidopsis thaliana) PIRL family in pollen development. However, for PIRL6, no bona fide knockout alleles have been recovered, suggesting that it may have an essential function in both male and female gametophytes. To test this hypothesis, we investigated PIRL6 expression and induced knockdown by RNA interference. Knockdown triggered defects in gametogenesis, resulting in abnormal pollen and early developmental arrest in the embryo sac. Consistent with this, PIRL6 was expressed in gametophytes: functional transcripts were detected in wild-type flowers but not in sporocyteless (spl) mutant flowers, which do not produce gametophytes. A genomic PIRL6-GFP fusion construct confirmed expression in both pollen and the embryo sac. Interestingly, PIRL6 is part of a convergent overlapping gene pair, a scenario associated with an increased likelihood of alternative splicing. We detected multiple alternative PIRL6 mRNAs in vegetative organs and spl mutant flowers, tissues that lacked the functionally spliced transcript. cDNA sequencing revealed that all contained intron sequences and premature termination codons. These alternative mRNAs accumulated in the nonsense-mediated decay mutant upf3, indicating that they are normally subjected to degradation. Together, these results demonstrate that PIRL6 is required in both male and female gametogenesis and suggest that sporophytic expression is negatively regulated by unproductive alternative splicing. This posttranscriptional mechanism may function to minimize PIRL6 protein expression in sporophyte tissues while allowing the overlapping adjacent gene to remain widely transcribed.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures





Similar articles
-
Unproductive alternative splicing and nonsense mRNAs: a widespread phenomenon among plant circadian clock genes.Biol Direct. 2012 Jul 2;7:20. doi: 10.1186/1745-6150-7-20. Biol Direct. 2012. PMID: 22747664 Free PMC article.
-
PHOSPHATIDYLSERINE SYNTHASE1 is required for microspore development in Arabidopsis thaliana.Plant J. 2011 Aug;67(4):648-61. doi: 10.1111/j.1365-313X.2011.04624.x. Epub 2011 Jun 24. Plant J. 2011. PMID: 21554450
-
PIRL1 and PIRL9, encoding members of a novel plant-specific family of leucine-rich repeat proteins, are essential for differentiation of microspores into pollen.Planta. 2010 Oct;232(5):1101-14. doi: 10.1007/s00425-010-1242-6. Epub 2010 Aug 10. Planta. 2010. PMID: 20697737
-
Phytosulfokine peptide signaling controls pollen tube growth and funicular pollen tube guidance in Arabidopsis thaliana.Physiol Plant. 2015 Apr;153(4):643-53. doi: 10.1111/ppl.12270. Epub 2014 Oct 8. Physiol Plant. 2015. PMID: 25174442
-
N-acetylglucosamine-1-P uridylyltransferase 1 and 2 are required for gametogenesis and embryo development in Arabidopsis thaliana.Plant Cell Physiol. 2014 Nov;55(11):1977-93. doi: 10.1093/pcp/pcu127. Epub 2014 Sep 16. Plant Cell Physiol. 2014. PMID: 25231969
Cited by
-
Unravelling Differential DNA Methylation Patterns in Genotype Dependent Manner under Salinity Stress Response in Chickpea.Int J Mol Sci. 2023 Jan 18;24(3):1863. doi: 10.3390/ijms24031863. Int J Mol Sci. 2023. PMID: 36768187 Free PMC article.
-
Combined Transcriptome Analysis Reveals the Ovule Abortion Regulatory Mechanisms in the Female Sterile Line of Pinus tabuliformis Carr.Int J Mol Sci. 2021 Mar 19;22(6):3138. doi: 10.3390/ijms22063138. Int J Mol Sci. 2021. PMID: 33808669 Free PMC article.
-
Expression analysis of plant intracellular Ras-group related leucine-rich repeat proteins (PIRLs) in Arabidopsis thaliana.Biochem Biophys Rep. 2022 Mar 5;30:101241. doi: 10.1016/j.bbrep.2022.101241. eCollection 2022 Jul. Biochem Biophys Rep. 2022. PMID: 35280522 Free PMC article.
-
RNA-seq analyses on gametogenic tissues of alfalfa (Medicago sativa) revealed plant reproduction- and ploidy-related genes.BMC Plant Biol. 2024 Sep 3;24(1):826. doi: 10.1186/s12870-024-05542-2. BMC Plant Biol. 2024. PMID: 39227784 Free PMC article.
-
Alternative Splicing Enhances the Transcriptome Complexity of Liriodendron chinense.Front Plant Sci. 2020 Sep 23;11:578100. doi: 10.3389/fpls.2020.578100. eCollection 2020. Front Plant Sci. 2020. PMID: 33072153 Free PMC article.
References
-
- Arciga-Reyes L, Wootton L, Kieffer M, Davies B (2006) UPF1 is required for nonsense-mediated mRNA decay (NMD) and RNAi in Arabidopsis. Plant J 47: 480–489 - PubMed
-
- Bencivenga S, Colombo L, Masiero S (2011) Cross talk between the sporophyte and the megagametophyte during ovule development. Sex Plant Reprod 24: 113–121 - PubMed
-
- Berg M, Rogers R, Muralla R, Meinke D (2005) Requirement of aminoacyl-tRNA synthetases for gametogenesis and embryo development in Arabidopsis. Plant J 44: 866–878 - PubMed
-
- Berger F, Twell D (2011) Germline specification and function in plants. Annu Rev Plant Biol 62: 461–484 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

