Development of an allosteric inhibitor class blocking RNA elongation by the respiratory syncytial virus polymerase complex
- PMID: 30206124
- PMCID: PMC6204889
- DOI: 10.1074/jbc.RA118.004862
Development of an allosteric inhibitor class blocking RNA elongation by the respiratory syncytial virus polymerase complex
Abstract
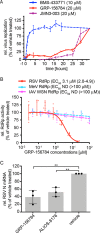
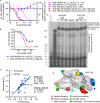
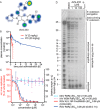
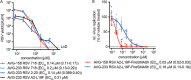
Respiratory syncytial virus (RSV) represents a significant health threat to infants and to elderly or immunocompromised individuals. There are currently no vaccines available to prevent RSV infections, and disease management is largely limited to supportive care, making the identification and development of effective antiviral therapeutics against RSV a priority. To identify effective chemical scaffolds for managing RSV disease, we conducted a high-throughput anti-RSV screen of a 57,000-compound library. We identified a hit compound that specifically blocked activity of the RSV RNA-dependent RNA polymerase (RdRp) complex, initially with moderate low-micromolar potency. Mechanistic characterization in an in vitro RSV RdRp assay indicated that representatives of this compound class block elongation of RSV RNA products after initial extension by up to three nucleotides. Synthetic hit-to-lead exploration yielded an informative 3D quantitative structure-activity relationship (3D-QSAR) model and resulted in analogs with more than 20-fold improved potency and selectivity indices (SIs) of >1,000. However, first-generation leads exhibited limited water solubility and poor metabolic stability. A second optimization strategy informed by the 3D-QSAR model combined with in silico pharmacokinetics (PK) predictions yielded an advanced lead, AVG-233, that demonstrated nanomolar activity against both laboratory-adapted RSV strains and clinical RSV isolates. This anti-RSV activity extended to infection of established cell lines and primary human airway cells. PK profiling in mice revealed 34% oral bioavailability of AVG-233 and sustained high drug levels in the circulation after a single oral dose of 20 mg/kg. This promising first-in-class lead warrants further development as an anti-RSV drug.
Keywords: RNA-dependent RNA polymerase; RdRp; animal virus; antiviral agent; drug development; medicinal chemistry; respiratory disease; respiratory syncytial virus; viral polymerase.
© 2018 Cox et al.
Conflict of interest statement
J. P., E. L., J. V., and R. K. P. are co-inventors on a patent application covering the use of the AVG-233 compound class for antiviral therapy. This study could affect their personal financial status
Figures




Similar articles
-
Preclinical Characterization of PC786, an Inhaled Small-Molecule Respiratory Syncytial Virus L Protein Polymerase Inhibitor.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e00737-17. doi: 10.1128/AAC.00737-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28652242 Free PMC article.
-
Discovery of a non-nucleoside inhibitor that binds to a novel site in the palm domain of the respiratory syncytial virus RNA-dependent RNA polymerase.J Virol. 2025 Jul 22;99(7):e0017825. doi: 10.1128/jvi.00178-25. Epub 2025 Jun 2. J Virol. 2025. PMID: 40454903 Free PMC article.
-
Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate.PLoS Pathog. 2015 Jun 22;11(6):e1004995. doi: 10.1371/journal.ppat.1004995. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26098424 Free PMC article.
-
New antiviral approaches for respiratory syncytial virus and other mononegaviruses: Inhibiting the RNA polymerase.Antiviral Res. 2016 Oct;134:63-76. doi: 10.1016/j.antiviral.2016.08.006. Epub 2016 Aug 27. Antiviral Res. 2016. PMID: 27575793 Review.
-
Respiratory syncytial virus entry inhibitors targeting the F protein.Viruses. 2013 Jan 16;5(1):211-25. doi: 10.3390/v5010211. Viruses. 2013. PMID: 23325327 Free PMC article. Review.
Cited by
-
Conserved allosteric inhibitory site on the respiratory syncytial virus and human metapneumovirus RNA-dependent RNA polymerases.Commun Biol. 2023 Jun 19;6(1):649. doi: 10.1038/s42003-023-04990-0. Commun Biol. 2023. PMID: 37337079 Free PMC article.
-
Facile Synthesis of Homodimeric Protein Ligands.Chembiochem. 2023 Sep 15;24(18):e202300392. doi: 10.1002/cbic.202300392. Epub 2023 Aug 17. Chembiochem. 2023. PMID: 37449865 Free PMC article.
-
Orally efficacious lead of the AVG inhibitor series targeting a dynamic interface in the respiratory syncytial virus polymerase.Sci Adv. 2022 Jun 24;8(25):eabo2236. doi: 10.1126/sciadv.abo2236. Epub 2022 Jun 24. Sci Adv. 2022. PMID: 35749502 Free PMC article.
-
Immune-Modulation by the Human Respiratory Syncytial Virus: Focus on Dendritic Cells.Front Immunol. 2019 Apr 15;10:810. doi: 10.3389/fimmu.2019.00810. eCollection 2019. Front Immunol. 2019. PMID: 31057543 Free PMC article. Review.
-
Structural Insight into Paramyxovirus and Pneumovirus Entry Inhibition.Viruses. 2020 Mar 20;12(3):342. doi: 10.3390/v12030342. Viruses. 2020. PMID: 32245118 Free PMC article. Review.
References
-
- Afonso C. L., Amarasinghe G. K., Bányai K., Bào Y., Basler C. F., Bavari S., Bejerman N., Blasdell K. R., Briand F. X., Briese T., Bukreyev A., Calisher C. H., Chandran K., Chéng J., Clawson A. N., et al. (2016) Taxonomy of the order Mononegavirales: update 2016. Arch. Virol. 161, 2351–2360 10.1007/s00705-016-2880-1 - DOI - PMC - PubMed
-
- Nair H., Nokes D. J., Gessner B. D., Dherani M., Madhi S. A., Singleton R. J., O'Brien K. L., Roca A., Wright P. F., Bruce N., Chandran A., Theodoratou E., Sutanto A., Sedyaningsih E. R., Ngama M., et al. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375, 1545–1555 10.1016/S0140-6736(10)60206-1 - DOI - PMC - PubMed
-
- Shi T., McAllister D. A., O'Brien K. L., Simoes E. A. F., Madhi S. A., Gessner B. D., Polack F. P., Balsells E., Acacio S., Aguayo C., Alassani I., Ali A., Antonio M., Awasthi S., Awori J. O., et al. (2017) Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390, 946–958 10.1016/S0140-6736(17)30938-8 - DOI - PMC - PubMed
-
- Lugo R. A., and Nahata M. C. (1993) Pathogenesis and treatment of bronchiolitis. Clin. Pharm. 12, 95–116 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

