Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke
- PMID: 30206183
- PMCID: PMC6202192
- DOI: 10.1136/tobaccocontrol-2018-054325
Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke
Abstract
Background: Heated tobacco products (also called 'heat-not-burn' products) heat tobacco at temperatures below that of combustion, causing nicotine and other compounds to aerosolise. One such product, IQOS from Philip Morris International, is being marketed internationally with claims of harm reduction. We sought to determine whether exposure to IQOS aerosol impairs arterial flow-mediated dilation (FMD), a measure of vascular endothelial function that is impaired by tobacco smoke.
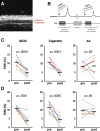
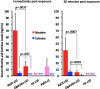
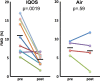
Methods: We exposed anaesthetised rats (n=8/group) via nose cone to IQOS aerosol from single HeatSticks, mainstream smoke from single Marlboro Red cigarettes or clean air for a series of consecutive 30 s cycles over 1.5-5 min. Each cycle consisted of 15 or 5 s of exposure followed by removal from the nose cone. We measured pre-exposure and postexposure FMD, and postexposure serum nicotine and cotinine.
Results: FMD was impaired comparably by ten 15 s exposures and ten 5 s exposures to IQOS aerosol and to cigarette smoke, but not by clean air. Serum nicotine levels were similar to plasma levels after humans have smoked one cigarette, confirming that exposure conditions had real-world relevance. Postexposure nicotine levels were ~4.5-fold higher in rats exposed to IQOS than to cigarettes, despite nicotine being measured in the IQOS aerosol at ~63% the amount measured in smoke. When IQOS exposure was briefer, leading to comparable serum nicotine levels to the cigarette group, FMD was still comparably impaired.
Conclusions: Acute exposures to IQOS aerosol impairs FMD in rats. IQOS use does not necessarily avoid the adverse cardiovascular effects of smoking cigarettes.
Keywords: electronic nicotine delivery devices; harm reduction; nicotine; non-cigarette tobacco products; smoking caused disease.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Heat-not-burn tobacco products: an emerging threat to cardiovascular health.Am J Physiol Heart Circ Physiol. 2020 Dec 1;319(6):H1234-H1239. doi: 10.1152/ajpheart.00708.2020. Epub 2020 Oct 2. Am J Physiol Heart Circ Physiol. 2020. PMID: 33006919 Free PMC article. Review.
-
Comparable Impairment of Vascular Endothelial Function by a Wide Range of Electronic Nicotine Delivery Devices.Nicotine Tob Res. 2022 Jun 15;24(7):1055-1062. doi: 10.1093/ntr/ntac019. Nicotine Tob Res. 2022. PMID: 35100430 Free PMC article.
-
IQOS: examination of Philip Morris International's claim of reduced exposure.Tob Control. 2018 Nov;27(Suppl 1):s30-s36. doi: 10.1136/tobaccocontrol-2018-054321. Epub 2018 Aug 29. Tob Control. 2018. PMID: 30158205 Free PMC article.
-
Free-Base and Total Nicotine, Reactive Oxygen Species, and Carbonyl Emissions From IQOS, a Heated Tobacco Product.Nicotine Tob Res. 2019 Aug 19;21(9):1285-1288. doi: 10.1093/ntr/nty235. Nicotine Tob Res. 2019. PMID: 30476301 Free PMC article.
-
IQOS - a heat-not-burn (HnB) tobacco product - chemical composition and possible impact on oxidative stress and inflammatory response. A systematic review.Toxicol Mech Methods. 2020 Feb;30(2):81-87. doi: 10.1080/15376516.2019.1669245. Epub 2019 Oct 2. Toxicol Mech Methods. 2020. PMID: 31532297
Cited by
-
PMI's own in vivo clinical data on biomarkers of potential harm in Americans show that IQOS is not detectably different from conventional cigarettes.Tob Control. 2018 Nov;27(Suppl 1):s9-s12. doi: 10.1136/tobaccocontrol-2018-054413. Epub 2018 Aug 21. Tob Control. 2018. PMID: 30131374 Free PMC article.
-
Impact of Heated Tobacco Products, E-Cigarettes, and Cigarettes on Inflammation and Endothelial Dysfunction.Int J Mol Sci. 2023 May 29;24(11):9432. doi: 10.3390/ijms24119432. Int J Mol Sci. 2023. PMID: 37298381 Free PMC article. Clinical Trial.
-
Effects of unburned tobacco smoke on inflammatory and oxidative mediators in the rat prefrontal cortex.Front Pharmacol. 2024 Jan 25;15:1328917. doi: 10.3389/fphar.2024.1328917. eCollection 2024. Front Pharmacol. 2024. PMID: 38333013 Free PMC article.
-
Heat-not-burn tobacco products: an emerging threat to cardiovascular health.Am J Physiol Heart Circ Physiol. 2020 Dec 1;319(6):H1234-H1239. doi: 10.1152/ajpheart.00708.2020. Epub 2020 Oct 2. Am J Physiol Heart Circ Physiol. 2020. PMID: 33006919 Free PMC article. Review.
-
Genome-wide differential expression profiling of lncRNAs and mRNAs in human induced pluripotent stem cell-derived endothelial cells exposed to e-cigarette extract.Stem Cell Res Ther. 2021 Dec 4;12(1):593. doi: 10.1186/s13287-021-02654-6. Stem Cell Res Ther. 2021. PMID: 34863290 Free PMC article.
References
-
- Philip Morris International. Designing a smoke-free future. https://www.pmi.com (accessed 24 Jan 2018).
-
- U.S. Food and Drug Administration. Philip Morris products S.A. Modified Risk Tobacco Product (MRTP) Applications. https://www.fda.gov/TobaccoProducts/Labeling/MarketingandAdvertising/ucm...
-
- Zenzen V, Diekmann J, Gerstenberg B, et al. . Reduced exposure evaluation of an Electrically Heated Cigarette Smoking System. Part 2: Smoke chemistry and in vitro toxicological evaluation using smoking regimens reflecting human puffing behavior. Regul Toxicol Pharmacol 2012;64(Suppl):S11–34. 10.1016/j.yrtph.2012.08.004 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
