Gastric cancer mesenchymal stem cells derived IL-8 induces PD-L1 expression in gastric cancer cells via STAT3/mTOR-c-Myc signal axis
- PMID: 30206229
- PMCID: PMC6134105
- DOI: 10.1038/s41419-018-0988-9
Gastric cancer mesenchymal stem cells derived IL-8 induces PD-L1 expression in gastric cancer cells via STAT3/mTOR-c-Myc signal axis
Abstract
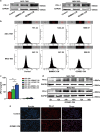
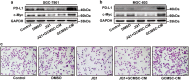
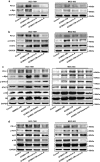
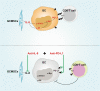
The expression of PD-L1 in tumor cells is one of the main causes of tumor immune escape. However, the exact mechanism for regulating PD-L1 expression in gastric cancer (GC) cells remains unclear. Our previous studies have shown that mesenchymal stem cells (MSCs) exert broad immunosuppressive potential, modulating the activity of cells either in innate or adaptive immune system to promote tumor progress. This study aims to investigate whether GCMSCs regulate the PD-L1 expression in GC cells and explore the specific molecular mechanism. The results have shown that GCMSCs enhanced PD-L1 expression in GC cells resulting in the resistance of GC cells to CD8+ T cells cytotoxicity. However, this resistance was attenuated with IL-8 inhibition. Further studies proved that IL-8 derived from GCMSCs induced PD-L1 expression in GC cells via c-Myc regulated by STAT3 and mTOR signaling pathways. Our data indicated that blocking IL-8 derived from GCMSCs may overcome the immune escape induced by PD-L1 in GC cells and provide a potential strategy to enhance the immunotherapy efficiency in GC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Gastric cancer mesenchymal stem cells regulate PD-L1-CTCF enhancing cancer stem cell-like properties and tumorigenesis.Theranostics. 2020 Oct 25;10(26):11950-11962. doi: 10.7150/thno.49717. eCollection 2020. Theranostics. 2020. PMID: 33204322 Free PMC article.
-
Gastric cancer mesenchymal stem cells via the CXCR2/HK2/PD-L1 pathway mediate immunosuppression.Gastric Cancer. 2023 Sep;26(5):691-707. doi: 10.1007/s10120-023-01405-1. Epub 2023 Jun 10. Gastric Cancer. 2023. PMID: 37300724
-
[Conditional medium of gastric cancer mesenchymal stem cells promotes PD-L1 expression in gastric cancer cells and tumor growth via upregulating VGLL4].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022 Apr;38(4):321-327. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022. PMID: 35583061 Chinese.
-
Recent advances in the mechanisms of PD-L1 expression in gastric cancer: a review.Biol Res. 2025 Mar 17;58(1):16. doi: 10.1186/s40659-025-00597-3. Biol Res. 2025. PMID: 40091086 Free PMC article. Review.
-
Research advances in the role of gastric cancer‑derived mesenchymal stem cells in tumor progression (Review).Int J Mol Med. 2021 Feb;47(2):455-462. doi: 10.3892/ijmm.2020.4810. Epub 2020 Dec 4. Int J Mol Med. 2021. PMID: 33416102 Review.
Cited by
-
Escherichia coli K12 Upregulates Programmed Cell Death Ligand 1 (PD-L1) Expression in Gamma Interferon-Sensitized Intestinal Epithelial Cells via the NF-κB Pathway.Infect Immun. 2020 Dec 15;89(1):e00618-20. doi: 10.1128/IAI.00618-20. Print 2020 Dec 15. Infect Immun. 2020. PMID: 33046511 Free PMC article.
-
STAT3 Pathway in Gastric Cancer: Signaling, Therapeutic Targeting and Future Prospects.Biology (Basel). 2020 Jun 12;9(6):126. doi: 10.3390/biology9060126. Biology (Basel). 2020. PMID: 32545648 Free PMC article. Review.
-
Tumour-associated macrophages: versatile players in the tumour microenvironment.Front Cell Dev Biol. 2023 Oct 26;11:1261749. doi: 10.3389/fcell.2023.1261749. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37965573 Free PMC article. Review.
-
Activation of SphK2 contributes to adipocyte-induced EOC cell proliferation.Open Med (Wars). 2022 Jan 31;17(1):229-238. doi: 10.1515/med-2022-0422. eCollection 2022. Open Med (Wars). 2022. PMID: 35178477 Free PMC article.
-
Tumor metastasis: Mechanistic insights and therapeutic interventions.MedComm (2020). 2021 Dec 2;2(4):587-617. doi: 10.1002/mco2.100. eCollection 2021 Dec. MedComm (2020). 2021. PMID: 34977870 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

