GWAS for Interleukin-1β levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation
- PMID: 30206230
- PMCID: PMC6134146
- DOI: 10.1038/s41467-018-05940-9
GWAS for Interleukin-1β levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation
Abstract
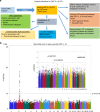
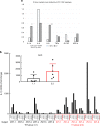
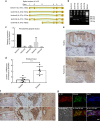
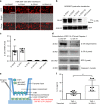
There is no agnostic GWAS evidence for the genetic control of IL-1β expression in periodontal disease. Here we report a GWAS for "high" gingival crevicular fluid IL-1β expression among 4910 European-American adults and identify association signals in the IL37 locus. rs3811046 at this locus (p = 3.3 × 10-22) is associated with severe chronic periodontitis (OR = 1.50; 95% CI = 1.12-2.00), 10-year incident tooth loss (≥3 teeth: RR = 1.33; 95% CI = 1.09-1.62) and aggressive periodontitis (OR = 1.12; 95% CI = 1.01-1.26) in an independent sample of 4927 German/Dutch adults. The minor allele at rs3811046 is associated with increased expression of IL-1β in periodontal tissue. In RAW macrophages, PBMCs and transgenic mice, the IL37 variant increases expression of IL-1β and IL-6, inducing more severe periodontal disease, while IL-37 protein production is impaired and shows reduced cleavage by caspase-1. A second variant in the IL37 locus (rs2708943, p = 4.2 × 10-7) associates with attenuated IL37 mRNA expression. Overall, we demonstrate that IL37 variants modulate the inflammatory cascade in periodontal disease.
Conflict of interest statement
Dr. Cowley is employed by, has equity ownership in, and serves on the board of directors of TransViragen, the company which has been contracted by UNC-Chapel Hill to manage its Animal Models Core Facility. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- R01 HL059367/HL/NHLBI NIH HHS/United States
- HHSN268201100011I/HL/NHLBI NIH HHS/United States
- U01 HG004402/HG/NHGRI NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- R01 DE023836/DE/NIDCR NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
- R01 HL087641/HL/NHLBI NIH HHS/United States
- R01 DE021418/DE/NIDCR NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- R01 DE011551/DE/NIDCR NIH HHS/United States
- F32 DE026688/DE/NIDCR NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- T90 DE021986/DE/NIDCR NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- F32 DE026688/DE/NIDCR NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

