The voltage-dependence of MscL has dipolar and dielectric contributions and is governed by local intramembrane electric field
- PMID: 30206263
- PMCID: PMC6133944
- DOI: 10.1038/s41598-018-31945-x
The voltage-dependence of MscL has dipolar and dielectric contributions and is governed by local intramembrane electric field
Abstract
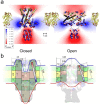
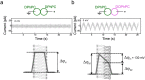
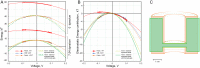
Channels without canonical voltage sensors can be modulated by voltage acting on other domains. Here we show that besides protein dipoles, pore hydration can be affected by electric fields. In patches, both WT MscL and its V23T mutant show a decrease in the tension midpoint with hyperpolarization. The mutant exhibits a stronger parabolic dependence of transition energy on voltage, highly consistent with the favourable dielectric contribution from water filling the expanding pore. Purified V23T MscL in DPhPC droplet interface bilayers shows a similar voltage dependence. When reconstituted in an asymmetric DOPhPC/DPhPC bilayer carrying a permanent bias of ~130 mV due to a dipole potential difference between the interfaces, the channel behaved as if the local intramembrane electric field sets the tension threshold for gating rather than just the externally applied voltage. The data emphasize the roles of polarized water in the pore and interfacial lipid dipoles in channel gating thermodynamics.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Properties of diphytanoyl phospholipids at the air-water interface.Langmuir. 2015;31(1):350-7. doi: 10.1021/la503800g. Epub 2014 Dec 18. Langmuir. 2015. PMID: 25474305
-
Activation of bacterial channel MscL in mechanically stimulated droplet interface bilayers.Sci Rep. 2015 Sep 8;5:13726. doi: 10.1038/srep13726. Sci Rep. 2015. PMID: 26348441 Free PMC article.
-
Electrophysiological interrogation of asymmetric droplet interface bilayers reveals surface-bound alamethicin induces lipid flip-flop.Biochim Biophys Acta Biomembr. 2019 Jan;1861(1):335-343. doi: 10.1016/j.bbamem.2018.07.001. Epub 2018 Jul 10. Biochim Biophys Acta Biomembr. 2019. PMID: 30006208
-
Asymmetric electrostatic effects on the gating of rat brain sodium channels in planar lipid membranes.Biophys J. 1991 Oct;60(4):845-55. doi: 10.1016/S0006-3495(91)82118-X. Biophys J. 1991. PMID: 1660316 Free PMC article.
-
Dipole potential of lipid membranes.Chem Phys Lipids. 1994 Sep 6;73(1-2):57-79. doi: 10.1016/0009-3084(94)90174-0. Chem Phys Lipids. 1994. PMID: 8001185 Review.
Cited by
-
Activating mechanosensitive channels embedded in droplet interface bilayers using membrane asymmetry.Chem Sci. 2021 Jan 4;12(6):2138-2145. doi: 10.1039/d0sc03889j. Chem Sci. 2021. PMID: 34163978 Free PMC article.
-
Dynamical nonlinear memory capacitance in biomimetic membranes.Nat Commun. 2019 Jul 19;10(1):3239. doi: 10.1038/s41467-019-11223-8. Nat Commun. 2019. PMID: 31324794 Free PMC article.
-
MscS inactivation and recovery are slow voltage-dependent processes sensitive to interactions with lipids.Biophys J. 2024 Jan 16;123(2):195-209. doi: 10.1016/j.bpj.2023.12.007. Epub 2023 Dec 14. Biophys J. 2024. PMID: 38098232 Free PMC article.
-
When is a hydrophobic gate not a hydrophobic gate?J Gen Physiol. 2022 Nov 7;154(11):e202213210. doi: 10.1085/jgp.202213210. Epub 2022 Oct 26. J Gen Physiol. 2022. PMID: 36287215 Free PMC article.
-
Decisive structural elements in water and ion permeation through mechanosensitive channels of large conductance: insights from molecular dynamics simulation.RSC Adv. 2022 Jun 16;12(28):17803-17816. doi: 10.1039/d2ra02284b. eCollection 2022 Jun 14. RSC Adv. 2022. PMID: 35765322 Free PMC article.
References
Publication types
Grants and funding
- FA9550-12-1-0464/DOD | Air Force Office of Scientific Research (AF Office of Scientific Research)/International
- FA9550-12-1-0464/DOD | Air Force Office of Scientific Research (AF Office of Scientific Research)/International
- FA9550-12-1-0464/DOD | Air Force Office of Scientific Research (AF Office of Scientific Research)/International
- FA9550-12-1-0464/DOD | Air Force Office of Scientific Research (AF Office of Scientific Research)/International
- FA9550-12-1-0464/DOD | Air Force Office of Scientific Research (AF Office of Scientific Research)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

