Matched Short-Term Depression and Recovery Encodes Interspike Interval at a Central Synapse
- PMID: 30206296
- PMCID: PMC6134063
- DOI: 10.1038/s41598-018-31996-0
Matched Short-Term Depression and Recovery Encodes Interspike Interval at a Central Synapse
Abstract
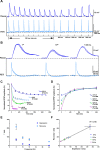
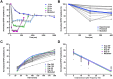
Reversible decreases in synaptic strength, known as short-term depression (STD), are widespread in neural circuits. Various computational roles have been attributed to STD but these tend to focus upon the initial depression rather than the subsequent recovery. We studied the role of STD and recovery at an excitatory synapse between the fast extensor tibiae (FETi) and flexor tibiae (flexor) motor neurons in the desert locust (Schistocerca gregaria) by making paired intracellular recordings in vivo. Over behaviorally relevant pre-synaptic spike frequencies, we found that this synapse undergoes matched frequency-dependent STD and recovery; higher frequency spikes that evoke stronger, faster STD also produce stronger, faster recovery. The precise matching of depression and recovery time constants at this synapse ensures that flexor excitatory post-synaptic potential (EPSP) amplitude encodes the presynaptic FETi interspike interval (ISI). Computational modelling shows that this precise matching enables the FETi-flexor synapse to linearly encode the ISI in the EPSP amplitude, a coding strategy that may be widespread in neural circuits.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

