Effects of dichloroacetate as single agent or in combination with GW6471 and metformin in paraganglioma cells
- PMID: 30206358
- PMCID: PMC6134030
- DOI: 10.1038/s41598-018-31797-5
Effects of dichloroacetate as single agent or in combination with GW6471 and metformin in paraganglioma cells
Abstract
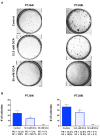
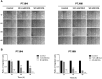
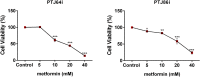
Paragangliomas (PGLs) are infiltrating autonomic nervous system tumors that cause important morbidity. At present, surgery is the only effective therapeutic option for this rare tumor. Thus, new agents for PGL treatment should be identified. Using unique PGL cell models established in our laboratory, we evaluated the effect of dichloroacetate (DCA) as single agent or in a novel combination with other metabolic drugs, including GW6471 and metformin. DCA and metformin had not been tested before in PGL. DCA reduced PGL cell viability and growth through mechanisms involving reactivation of PDH complex leading to promotion of oxidative metabolism, with lowering of lactate and enhanced ROS production. This resulted in cell cycle inhibition and induction of apoptosis in PGL cells, as shown by flow cytometry and immunoblot analyses. Moreover, DCA drastically impaired clonogenic activity and migration of PGL cells. Also metformin reduced PGL cell viability as single agent and the combinations of DCA, GW6471 and metformin had strong effects on cell viability. Furthermore, combined treatments had drastic and synergistic effects on clonogenic ability. In conclusion, DCA, GW6471 and metformin as single agents and in combination appear to have promising antitumor effects in unique cell models of PGL.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

