A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L
- PMID: 30206369
- PMCID: PMC6173687
- DOI: 10.1038/s41416-018-0243-2
A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L
Abstract
Background: Cisplatin-based chemoradiotherapy is the standard treatment for unresectable, locally advanced non-small-cell lung cancer (NSCLC). This trial evaluated two experimental regimens that combine chemotherapy with concurrent radiotherapy.
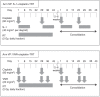
Methods: Eligible patients with unresectable stage III NSCLC were randomised to either the SP arm (S-1 and cisplatin) or VP arm (vinorelbine and cisplatin), with early concurrent thoracic radiotherapy of 60 Gy, comprising 2 Gy per daily fraction. The primary endpoint was the overall survival rate at 2 years (2-year overall survival (OS)) (Study ID: UMIN000002420).
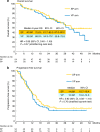
Results: From September 2009 to September 2012, 112 patients were enroled. Of the 108 eligible patients, the 2-year OS was 75.6% (80% confidence interval (CI), 67-82%) in the SP arm and 68.5% (80% CI: 60-76%) in the VP arm. The hazard ratio (HR) for death between the two arms was 0.85 (0.48-1.49). The median progression-free survival was 14.8 months for the SP arm and 12.3 months for the VP arm with an HR of 0.92 (0.58-1.44). There were four treatment-related deaths in the SP arm and five in the VP arm.
Conclusions: The null hypotheses for 2-year OS were rejected in both arms. The West Japan Oncology Group will employ the SP arm as the investigational arm in a future phase III study.
Conflict of interest statement
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. T. Seto has received honoraria from Kyowa Hakko Kirin Co., Ltd. and Taiho Pharmaceutical Co., Ltd. T.Y. has received personal fees from Taiho Pharmaceutical Co., Ltd. N.K. has received personal fees from Taiho Pharmaceutical Co., Ltd. M.N. has received grants and personal fees from Taiho Pharmaceutical Co., Ltd. T.T. has received grants and personal fees from Taiho Pharmaceutical Co., Ltd. Y.I. has received honoraria from Kyowa Hakko Kirin Co., Ltd. and Taiho Pharmaceutical Co., Ltd. K.N. has received grants and personal fees from Taiho Pharmaceutical Co., Ltd., during the conduct of the study; grants and personal fees from Kyowa Hakko Kirin Co., Ltd. N. Yamamoto has received grants and personal fees from Kyowa Hakko Kirin Co., Ltd. and Taiho Pharmaceutical Co., Ltd. Y. Nakanishi has received grants from Taiho Pharmaceutical Co., Ltd. All other authors declared no competing interests.
Figures

Similar articles
-
Randomized phase II trial of S-1 plus cisplatin or docetaxel plus cisplatin with concurrent thoracic radiotherapy for inoperable stage III non-small cell lung cancer.Cancer Med. 2021 Jan;10(2):626-633. doi: 10.1002/cam4.3641. Epub 2020 Dec 14. Cancer Med. 2021. PMID: 33319495 Free PMC article. Clinical Trial.
-
S-1 plus cisplatin with concurrent radiotherapy for locally advanced non-small cell lung cancer: a multi-institutional phase II trial (West Japan Thoracic Oncology Group 3706).J Thorac Oncol. 2011 Dec;6(12):2069-75. doi: 10.1097/JTO.0b013e3182307e5a. J Thorac Oncol. 2011. PMID: 22052226 Clinical Trial.
-
Long-term results of S-1 plus cisplatin with concurrent thoracic radiotherapy for locally advanced non-small-cell lung cancer.Cancer Chemother Pharmacol. 2018 Mar;81(3):565-572. doi: 10.1007/s00280-018-3530-y. Epub 2018 Jan 31. Cancer Chemother Pharmacol. 2018. PMID: 29387962 Clinical Trial.
-
Oral vinorelbine-based concomitant chemoradiotherapy in unresectable stage III non-small cell lung cancer: a systematic review.Expert Rev Anticancer Ther. 2018 Nov;18(11):1159-1165. doi: 10.1080/14737140.2018.1518714. Epub 2018 Sep 17. Expert Rev Anticancer Ther. 2018. PMID: 30173589
-
Clinical studies in non-small cell lung cancer: the CALGB experience.Cancer Invest. 1998;16(2):72-9. doi: 10.3109/07357909809039760. Cancer Invest. 1998. PMID: 9512672 Review.
Cited by
-
Safety and efficacy of neoadjuvant cisplatin + S-1 combined with radiation therapy for locally advanced non-small cell lung cancer.Surg Today. 2025 Jul;55(7):886-899. doi: 10.1007/s00595-025-03019-9. Epub 2025 Feb 27. Surg Today. 2025. PMID: 40014076 Free PMC article.
-
A phase I study of S-1 and cisplatin with concurrent hypofractionated carbon-ion radiotherapy for patients with stage III non-small cell lung cancer.Front Oncol. 2025 Aug 5;15:1573462. doi: 10.3389/fonc.2025.1573462. eCollection 2025. Front Oncol. 2025. PMID: 40837011 Free PMC article.
-
Consolidation Systemic Therapy in Locally Advanced, Inoperable Nonsmall Cell Lung Cancer-How to Identify Patients Which Can Benefit from It?Curr Oncol. 2022 Oct 31;29(11):8316-8329. doi: 10.3390/curroncol29110656. Curr Oncol. 2022. PMID: 36354716 Free PMC article. Review.
-
A New Sensitive Method for Quantitative Determination of Cisplatin in Biological Samples by Kinetic Spectrophotometry.Anal Sci. 2020 Oct 10;36(10):1217-1221. doi: 10.2116/analsci.20P118. Epub 2020 May 15. Anal Sci. 2020. PMID: 32418934
-
Population Survival Kinetics Derived from Clinical Trials of Potentially Curable Lung Cancers.Curr Oncol. 2024 Mar 20;31(3):1600-1617. doi: 10.3390/curroncol31030122. Curr Oncol. 2024. PMID: 38534955 Free PMC article.
References
-
- Segawa Y, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J. Clin. Oncol. 2010;28:3299–3306. doi: 10.1200/JCO.2009.24.7577. - DOI - PubMed
-
- Yamamoto N, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. - DOI - PubMed
-
- Shirasaka T, et al. Antitumor activity of 1 M tegafur–0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602–2606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

