Antimicrobial action of calprotectin that does not involve metal withholding
- PMID: 30206620
- PMCID: PMC6417507
- DOI: 10.1039/c8mt00133b
Antimicrobial action of calprotectin that does not involve metal withholding
Abstract
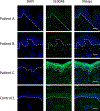
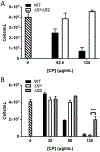
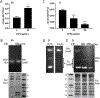
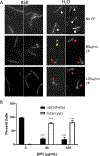
Calprotectin is a potent antimicrobial that inhibits the growth of pathogens by tightly binding transition metals such as Mn and Zn, thereby preventing their uptake and utilization by invading microbes. At sites of infection, calprotectin is abundantly released from neutrophils, but calprotectin is also present in non-neutrophil cell types that may be relevant to infections. We show here that in patients infected with the Lyme disease pathogen Borreliella (Borrelia) burgdorferi, calprotectin is produced in neutrophil-free regions of the skin, in both epidermal keratinocytes and in immune cells infiltrating the dermis, including CD68 positive macrophages. In culture, B. burgdorferi's growth is inhibited by calprotectin, but surprisingly, the mechanism does not involve the classical withholding of metal nutrients. B. burgdorferi cells exposed to calprotectin cease growth with no reduction in intracellular Mn and no loss in activity of Mn enzymes including the SodA superoxide dismutase. Additionally, there is no obvious loss in intracellular Zn. Rather than metal depletion, we find that calprotectin inhibits B. burgdorferi growth through a mechanism that requires physical association of calprotectin with the bacteria. By comparison, calprotectin inhibited E. coli growth without physically interacting with the microbe, and calprotectin effectively depleted E. coli of intracellular Mn and Zn. Our studies with B. burgdorferi demonstrate that the antimicrobial capacity of calprotectin is complex and extends well beyond simple withholding of metal micronutrients.
Conflict of interest statement
CONFLICTS OF INTEREST
There are no conflicts of interest to declare.
Figures







References
-
- Vogl T, Leukert N, Barczyk K, Strupat K and Roth J, Biophysical characterization of S100A8 and S100A9 in the absence and presence of bivalent cations, Biochim. Biophys. Acta, 2006, 1763, 1298–1306. - PubMed
-
- Strupat K, Rogniaux H, Van Dorsselaer A, Roth J and Vogl T, Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis, J. Am. Soc. Mass Spectrom, 2000, 11, 780–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

