Complex and spatially segregated auditory inputs of the mouse superior colliculus
- PMID: 30206945
- PMCID: PMC6209754
- DOI: 10.1113/JP276370
Complex and spatially segregated auditory inputs of the mouse superior colliculus
Abstract
Key points: Although the visual circuits in the superior colliculus (SC) have been thoroughly examined, the auditory circuits lack equivalent scrutiny. SC neurons receiving auditory inputs in mice were characterized and three distinguishable types of neurons were found. The auditory pathways from external nuclei of the inferior colliculus (IC) were characterized, and a novel direct inhibitory connection and an excitation that drives feed-forward inhibitory circuits within the SC were found. The direct excitatory and inhibitory inputs exhibited distinct arbourization patterns in the SC. These findings suggest functional differences between excitatory and inhibitory sensory information that targets the auditory SC.
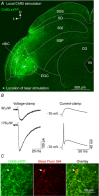
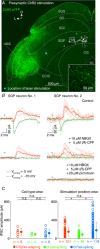
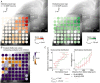
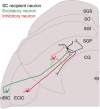
Abstract: The superior colliculus (SC) is a midbrain structure that integrates auditory, somatosensory and visual inputs to drive orientation movements. While much is known about how visual information is processed in the superficial layers of the SC, little is known about the SC circuits in the deep layers that process auditory inputs. We therefore characterized intrinsic neuronal properties in the auditory-recipient layer of the SC (stratum griseum profundum; SGP) and confirmed three electrophysiologically defined clusters of neurons, consistent with literature from other SC layers. To determine the types of inputs to the SGP, we expressed Channelrhodopsin-2 in the nucleus of the brachium of the inferior colliculus (nBIC) and external cortex of the inferior colliculus (ECIC) and optically stimulated these pathways while recording from SGP neurons. Probing the connections in this manner, we described a monosynaptic excitation that additionally drives feed-forward inhibition via circuits intrinsic to the SC. Moreover, we found a profound long-range monosynaptic inhibition in 100% of recorded SGP neurons, a surprising finding considering that only about 15% of SGP-projecting neurons in the nBIC/ECIC are inhibitory. Furthermore, we found spatial differences in the cell body locations as well as axon trajectories between the monosynaptic excitatory and inhibitory inputs, suggesting that these inputs may be functionally distinct. Taking this together with recent anatomical evidence suggesting an auditory excitation from the nBIC and a GABAergic multimodal inhibition from the ECIC, we propose that sensory integration in the SGP is more multifaceted than previously thought.
Keywords: Auditory pathways; Mouse; Neural circuits; Superior colliculus.
© 2018 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures



References
-
- Aitkin LM & Phillips SC (1984). The interconnections of the inferior colliculi through their commissure. J Comp Neurol 228, 210–216. - PubMed
-
- Appell PP & Behan M (1990). Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol 302, 143–158. - PubMed
-
- Chevalier G & Mana S (2000). Honeycomb‐like structure of the intermediate layers of the rat superior colliculus, with additional observations in several other mammals: AChE patterning. J Comp Neurol 419, 137–153. - PubMed
-
- Covey E, Hall WC & Kobler JB (1987). Subcortical connections of the superior colliculus in the mustache bat, Pteronotus parnellii . J Comp Neurol 263, 179–197. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

