Geometric partitioning of cohesin and condensin is a consequence of chromatin loops
- PMID: 30207827
- PMCID: PMC6249845
- DOI: 10.1091/mbc.E18-02-0131
Geometric partitioning of cohesin and condensin is a consequence of chromatin loops
Abstract
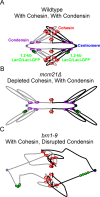
SMC (structural maintenance of chromosomes) complexes condensin and cohesin are crucial for proper chromosome organization. Condensin has been reported to be a mechanochemical motor capable of forming chromatin loops, while cohesin passively diffuses along chromatin to tether sister chromatids. In budding yeast, the pericentric region is enriched in both condensin and cohesin. As in higher-eukaryotic chromosomes, condensin is localized to the axial chromatin of the pericentric region, while cohesin is enriched in the radial chromatin. Thus, the pericentric region serves as an ideal model for deducing the role of SMC complexes in chromosome organization. We find condensin-mediated chromatin loops establish a robust chromatin organization, while cohesin limits the area that chromatin loops can explore. Upon biorientation, extensional force from the mitotic spindle aggregates condensin-bound chromatin from its equilibrium position to the axial core of pericentric chromatin, resulting in amplified axial tension. The axial localization of condensin depends on condensin's ability to bind to chromatin to form loops, while the radial localization of cohesin depends on cohesin's ability to diffuse along chromatin. The different chromatin-tethering modalities of condensin and cohesin result in their geometric partitioning in the presence of an extensional force on chromatin.
Figures




Similar articles
-
Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring.J Cell Biol. 2011 Jun 27;193(7):1167-80. doi: 10.1083/jcb.201103138. J Cell Biol. 2011. PMID: 21708976 Free PMC article.
-
The spatial segregation of pericentric cohesin and condensin in the mitotic spindle.Mol Biol Cell. 2013 Dec;24(24):3909-19. doi: 10.1091/mbc.E13-06-0325. Epub 2013 Oct 23. Mol Biol Cell. 2013. PMID: 24152737 Free PMC article.
-
SMC complexes differentially compact mitotic chromosomes according to genomic context.Nat Cell Biol. 2017 Sep;19(9):1071-1080. doi: 10.1038/ncb3594. Epub 2017 Aug 21. Nat Cell Biol. 2017. PMID: 28825700 Free PMC article.
-
The regulation of chromosome segregation via centromere loops.Crit Rev Biochem Mol Biol. 2019 Aug;54(4):352-370. doi: 10.1080/10409238.2019.1670130. Epub 2019 Oct 1. Crit Rev Biochem Mol Biol. 2019. PMID: 31573359 Free PMC article. Review.
-
Deciphering condensin action during chromosome segregation.Trends Cell Biol. 2011 Sep;21(9):552-9. doi: 10.1016/j.tcb.2011.06.003. Epub 2011 Jul 15. Trends Cell Biol. 2011. PMID: 21763138 Review.
Cited by
-
R-loops at centromeric chromatin contribute to defects in kinetochore integrity and chromosomal instability in budding yeast.Mol Biol Cell. 2021 Jan 1;32(1):74-89. doi: 10.1091/mbc.E20-06-0379. Epub 2020 Nov 4. Mol Biol Cell. 2021. PMID: 33147102 Free PMC article.
-
Common Features of the Pericentromere and Nucleolus.Genes (Basel). 2019 Dec 10;10(12):1029. doi: 10.3390/genes10121029. Genes (Basel). 2019. PMID: 31835574 Free PMC article. Review.
-
Polymer models reveal how chromatin modification can modulate force at the kinetochore.Mol Biol Cell. 2022 Sep 15;33(11):ar97. doi: 10.1091/mbc.E22-02-0041. Epub 2022 Jun 15. Mol Biol Cell. 2022. PMID: 35704466 Free PMC article.
-
Biochemical evidence for diverse strategies in the inner kinetochore.Open Biol. 2020 Nov;10(11):200284. doi: 10.1098/rsob.200284. Epub 2020 Nov 18. Open Biol. 2020. PMID: 33202170 Free PMC article. Review.
-
Force generation and resistance in human mitosis.Biophys Rev. 2024 Sep 28;16(5):551-562. doi: 10.1007/s12551-024-01235-0. eCollection 2024 Oct. Biophys Rev. 2024. PMID: 39618797 Free PMC article. Review.
References
-
- Cohen J. (1988). Statistical Power Analysis for the Behavioral Sciences, Hillsdale, NJ: L. Erlbaum Associates.
-
- Dewar H, Tanaka K, Nasmyth K, Tanaka TU. (2004). Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature , 93–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

