Tracking yeast pheromone receptor Ste2 endocytosis using fluorogen-activating protein tagging
- PMID: 30207829
- PMCID: PMC6249837
- DOI: 10.1091/mbc.E18-07-0424
Tracking yeast pheromone receptor Ste2 endocytosis using fluorogen-activating protein tagging
Abstract
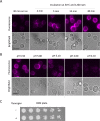
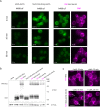
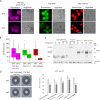
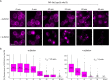
To observe internalization of the yeast pheromone receptor Ste2 by fluorescence microscopy in live cells in real time, we visualized only those molecules present at the cell surface at the time of agonist engagement (rather than the total cellular pool) by tagging this receptor at its N-terminus with an exocellular fluorogen-activating protein (FAP). A FAP is a single-chain antibody engineered to bind tightly a nonfluorescent, cell-impermeable dye (fluorogen), thereby generating a fluorescent complex. The utility of FAP tagging to study trafficking of integral membrane proteins in yeast, which possesses a cell wall, had not been examined previously. A diverse set of signal peptides and propeptide sequences were explored to maximize expression. Maintenance of the optimal FAP-Ste2 chimera intact required deletion of two, paralogous, glycosylphosphatidylinositol (GPI)-anchored extracellular aspartyl proteases (Yps1 and Mkc7). FAP-Ste2 exhibited a much brighter and distinct plasma membrane signal than Ste2-GFP or Ste2-mCherry yet behaved quite similarly. Using FAP-Ste2, new information was obtained about the mechanism of its internalization, including novel insights about the roles of the cargo-selective endocytic adaptors Ldb19/Art1, Rod1/Art4, and Rog3/Art7.
Figures
Similar articles
-
Specific α-arrestins negatively regulate Saccharomyces cerevisiae pheromone response by down-modulating the G-protein-coupled receptor Ste2.Mol Cell Biol. 2014 Jul;34(14):2660-81. doi: 10.1128/MCB.00230-14. Mol Cell Biol. 2014. PMID: 24820415 Free PMC article.
-
Differential Phosphorylation Provides a Switch to Control How α-Arrestin Rod1 Down-regulates Mating Pheromone Response in Saccharomyces cerevisiae.Genetics. 2016 May;203(1):299-317. doi: 10.1534/genetics.115.186122. Epub 2016 Feb 26. Genetics. 2016. PMID: 26920760 Free PMC article.
-
A syntaxin homolog encoded by VAM3 mediates down-regulation of a yeast G protein-coupled receptor.J Biol Chem. 1999 Jan 15;274(3):1835-41. doi: 10.1074/jbc.274.3.1835. J Biol Chem. 1999. PMID: 9880567
-
Fluorogen-Activating Proteins: Next-Generation Fluorescence Probes for Biological Research.Bioconjug Chem. 2020 Jan 15;31(1):16-27. doi: 10.1021/acs.bioconjchem.9b00710. Epub 2019 Dec 13. Bioconjug Chem. 2020. PMID: 31789501 Review.
-
Internalization and trafficking of fluorescent-labeled phospholipids in yeast.Semin Cell Dev Biol. 2002 Jun;13(3):179-84. doi: 10.1016/s1084-9521(02)00046-0. Semin Cell Dev Biol. 2002. PMID: 12137738 Review.
Cited by
-
Generation of endogenous pH-sensitive EGF receptor and its application in high-throughput screening for proteins involved in clathrin-mediated endocytosis.Elife. 2019 May 8;8:e46135. doi: 10.7554/eLife.46135. Elife. 2019. PMID: 31066673 Free PMC article.
-
Optimization of the fluorogen-activating protein tag for quantitative protein trafficking and colocalization studies in S. cerevisiae.Mol Biol Cell. 2024 Jul 1;35(7):mr5. doi: 10.1091/mbc.E24-04-0174. Epub 2024 May 29. Mol Biol Cell. 2024. PMID: 38809589 Free PMC article.
-
Comparison of Experimental Approaches Used to Determine the Structure and Function of the Class D G Protein-Coupled Yeast α-Factor Receptor.Biomolecules. 2022 May 30;12(6):761. doi: 10.3390/biom12060761. Biomolecules. 2022. PMID: 35740886 Free PMC article. Review.
-
TOR signaling regulates GPCR levels on the plasma membrane and suppresses the Saccharomyces cerevisiae mating pathway.bioRxiv [Preprint]. 2025 Apr 5:2024.05.09.593412. doi: 10.1101/2024.05.09.593412. bioRxiv. 2025. PMID: 38798445 Free PMC article. Preprint.
-
How Diffusion Impacts Cortical Protein Distribution in Yeasts.Cells. 2020 Apr 30;9(5):1113. doi: 10.3390/cells9051113. Cells. 2020. PMID: 32365827 Free PMC article. Review.
References
-
- Amberg DC, Burke DJ, Strathern JN. (2005). Methods in Yeast Genetics, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
-
- Arkowitz RA. (1999). Responding to attraction: chemotaxis and chemotropism in Dictyostelium and yeast. Trends Cell Biol , 20–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

