Vestibular and corticospinal control of human body orientation in the gravitational field
- PMID: 30207862
- PMCID: PMC6337031
- DOI: 10.1152/jn.00483.2018
Vestibular and corticospinal control of human body orientation in the gravitational field
Abstract
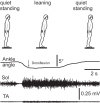
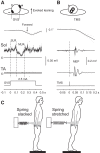
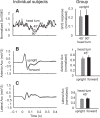
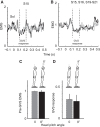
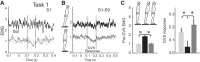
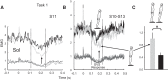
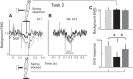
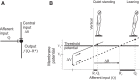
Body orientation with respect to the direction of gravity changes when we lean forward from upright standing. We tested the hypothesis that during upright standing, the nervous system specifies the referent body orientation that defines spatial thresholds for activation of multiple muscles across the body. To intentionally lean the body forward, the system is postulated to transfer balance and stability to the leaned position by monotonically tilting the referent orientation, thus increasing the activation thresholds of ankle extensors and decreasing their activity. Consequently, the unbalanced gravitational torque would start to lean the body forward. With restretching, ankle extensors would be reactivated and generate increasing electromyographic (EMG) activity until the enhanced gravitational torque would be balanced at a new posture. As predicted, vestibular influences on motoneurons of ankle extensors evaluated by galvanic vestibular stimulation were smaller in the leaned compared with the upright position, despite higher tonic EMG activity. Defacilitation of vestibular influences was also observed during forward leaning when the EMG levels in the upright and leaned position were equalized by compensating the gravitational torque with a load. The vestibular system is involved in the active control of body orientation without directly specifying the motor outcome. Corticospinal influences originating from the primary motor cortex evaluated by transcranial magnetic stimulation remained similar at the two body postures. Thus, in contrast to the vestibular system, the corticospinal system maintains a similar descending facilitation of motoneurons of leg muscles at different body orientations. The study advances the understanding of how body orientation is controlled. NEW & NOTEWORTHY The brain changes the referent body orientation with respect to gravity to lean the body forward. Physiologically, this is achieved by shifts in spatial thresholds for activation of ankle muscles, which involves the vestibular system. Results advance the understanding of how the brain controls body orientation in the gravitational field. The study also extends previous evidence of empirical control of motor function, i.e., without the reliance on model-based computations and direct specification of motor outcome.
Keywords: GVS; TMS; corticospinal; leaning; movement; posture; referent control; vestibular.
Figures


Similar articles
-
Assessment of vestibulocortical interactions during standing in healthy subjects.PLoS One. 2020 Jun 4;15(6):e0233843. doi: 10.1371/journal.pone.0233843. eCollection 2020. PLoS One. 2020. PMID: 32497147 Free PMC article.
-
Indirect, referent control of motor actions underlies directional tuning of neurons.J Neurophysiol. 2019 Mar 1;121(3):823-841. doi: 10.1152/jn.00575.2018. Epub 2018 Dec 19. J Neurophysiol. 2019. PMID: 30565957 Free PMC article. Review.
-
Referent control of the orientation of posture and movement in the gravitational field.Exp Brain Res. 2018 Feb;236(2):381-398. doi: 10.1007/s00221-017-5133-y. Epub 2017 Nov 21. Exp Brain Res. 2018. PMID: 29164285
-
Ancestral persistence of vestibulospinal reflexes in axial muscles in humans.J Neurophysiol. 2020 May 1;123(5):2010-2023. doi: 10.1152/jn.00421.2019. Epub 2020 Apr 22. J Neurophysiol. 2020. PMID: 32319843
-
Central pattern generator and human locomotion in the context of referent control of motor actions.Clin Neurophysiol. 2021 Nov;132(11):2870-2889. doi: 10.1016/j.clinph.2021.08.016. Epub 2021 Sep 27. Clin Neurophysiol. 2021. PMID: 34628342 Review.
Cited by
-
The 2022 On-site Padua Days on Muscle and Mobility Medicine hosts the University of Florida Institute of Myology and the Wellstone Center, March 30 - April 3, 2022 at the University of Padua and Thermae of Euganean Hills, Padua, Italy: The collection of abstracts.Eur J Transl Myol. 2022 Mar 10;32(1):10440. doi: 10.4081/ejtm.2022.10440. Eur J Transl Myol. 2022. PMID: 35272451 Free PMC article.
-
Assessment of vestibulocortical interactions during standing in healthy subjects.PLoS One. 2020 Jun 4;15(6):e0233843. doi: 10.1371/journal.pone.0233843. eCollection 2020. PLoS One. 2020. PMID: 32497147 Free PMC article.
-
Beyond rambling and trembling: effects of visual feedback on slow postural drift.Exp Brain Res. 2019 Mar;237(3):865-871. doi: 10.1007/s00221-019-05470-w. Epub 2019 Jan 11. Exp Brain Res. 2019. PMID: 30635703 Free PMC article.
-
Indirect, referent control of motor actions underlies directional tuning of neurons.J Neurophysiol. 2019 Mar 1;121(3):823-841. doi: 10.1152/jn.00575.2018. Epub 2018 Dec 19. J Neurophysiol. 2019. PMID: 30565957 Free PMC article. Review.
-
Efference copy in kinesthetic perception: a copy of what is it?J Neurophysiol. 2021 Apr 1;125(4):1079-1094. doi: 10.1152/jn.00545.2020. Epub 2021 Feb 10. J Neurophysiol. 2021. PMID: 33566734 Free PMC article. Review.
References
-
- Andronov AA, Vitt AA, Khaikin SE. Theory of Oscillators. New York: Dover, 2011.
-
- Asatryan DG, Feldman AG. Functional tuning of the nervous system with control of movements or maintenance of a steady posture: I. Mechanographic analysis of the work of the joint on execution of a postural task. Biophysics (Oxf) 10: 925–935, 1965.
-
- Auslander J, Bhatia NP, Seibert P. Attractors in dynamical systems. Bol Soc Mat Mex 9: 55–66, 1964.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

