Mutation Clearance after Transplantation for Myelodysplastic Syndrome
- PMID: 30207916
- PMCID: PMC6309244
- DOI: 10.1056/NEJMoa1804714
Mutation Clearance after Transplantation for Myelodysplastic Syndrome
Abstract
Background: Allogeneic hematopoietic stem-cell transplantation is the only curative treatment for patients with myelodysplastic syndrome (MDS). The molecular predictors of disease progression after transplantation are unclear.
Methods: We sequenced bone marrow and skin samples from 90 adults with MDS who underwent allogeneic hematopoietic stem-cell transplantation after a myeloablative or reduced-intensity conditioning regimen. We detected mutations before transplantation using enhanced exome sequencing, and we evaluated mutation clearance by using error-corrected sequencing to genotype mutations in bone marrow samples obtained 30 days after transplantation. In this exploratory study, we evaluated the association of a mutation detected after transplantation with disease progression and survival.
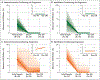
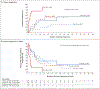
Results: Sequencing identified at least one validated somatic mutation before transplantation in 86 of 90 patients (96%); 32 of these patients (37%) had at least one mutation with a maximum variant allele frequency of at least 0.5% (equivalent to 1 heterozygous mutant cell in 100 cells) 30 days after transplantation. Patients with disease progression had mutations with a higher maximum variant allele frequency at 30 days than those who did not (median maximum variant allele frequency, 0.9% vs. 0%; P<0.001). The presence of at least one mutation with a variant allele frequency of at least 0.5% at day 30 was associated with a higher risk of progression (53.1% vs. 13.0%; conditioning regimen-adjusted hazard ratio, 3.86; 95% confidence interval [CI], 1.96 to 7.62; P<0.001) and a lower 1-year rate of progression-free survival than the absence of such a mutation (31.3% vs. 59.3%; conditioning regimen-adjusted hazard ratio for progression or death, 2.22; 95% CI, 1.32 to 3.73; P=0.005). The rate of progression-free survival was lower among patients who had received a reduced-intensity conditioning regimen and had at least one persistent mutation with a variant allele frequency of at least 0.5% at day 30 than among patients with other combinations of conditioning regimen and mutation status (P≤0.001). Multivariate analysis confirmed that patients who had a mutation with a variant allele frequency of at least 0.5% detected at day 30 had a higher risk of progression (hazard ratio, 4.48; 95% CI, 2.21 to 9.08; P<0.001) and a lower 1-year rate of progression-free survival than those who did not (hazard ratio for progression or death, 2.39; 95% CI, 1.40 to 4.09; P=0.002).
Conclusions: The risk of disease progression was higher among patients with MDS in whom persistent disease-associated mutations were detected in the bone marrow 30 days after transplantation than among those in whom these mutations were not detected. (Funded by the Leukemia and Lymphoma Society and others.).
Figures


Comment in
-
Mutation Clearance after Transplantation for Myelodysplastic Syndrome.N Engl J Med. 2018 Dec 13;379(24):2379. doi: 10.1056/NEJMc1813470. N Engl J Med. 2018. PMID: 30592585 No abstract available.
-
Mutation Clearance after Transplantation for Myelodysplastic Syndrome.N Engl J Med. 2018 Dec 13;379(24):2379-2380. doi: 10.1056/NEJMc1813470. N Engl J Med. 2018. PMID: 30592586 No abstract available.
References
-
- Koenecke C, Göhring G, de Wreede LC, et al. Impact of the revised International Prognostic Scoring System, cytogenetics and monosomal karyotype on outcome after allogeneic stem cell transplantation for myelodysplastic syndromes and secondary acute myeloid leukemia evolving from myelodysplastic syndromes: a retrospective multicenter study of the European Society of Blood and Marrow Transplantation. Haematologica 2015; 100: 400–8. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
