Transcription factor CBF-1 is critical for circadian gene expression by modulating WHITE COLLAR complex recruitment to the frq locus
- PMID: 30208021
- PMCID: PMC6152987
- DOI: 10.1371/journal.pgen.1007570
Transcription factor CBF-1 is critical for circadian gene expression by modulating WHITE COLLAR complex recruitment to the frq locus
Abstract
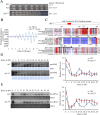
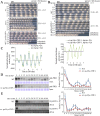
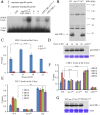
Transcription of the Neurospora crassa circadian clock gene frequency (frq) is an essential process in the negative feedback loop that controls circadian rhythms. WHITE COLLAR 1 (WC-1) and WHITE COLLAR 2 (WC-2) forms the WC complex (WCC) that is the main activator of frq transcription by binding to its promoter. Here, we show that Centromere Binding Factor 1 (CBF-1) is a critical component of the N. crassa circadian clock by regulating frq transcription. Deletion of cbf-1 resulted in long period and low amplitude rhythms, whereas overexpression of CBF-1 abolished the circadian rhythms. Loss of CBF-1 resulted in WC-independent FRQ expression and suppression of WCC activity. As WCC, CBF-1 also binds to the C-box at the frq promoter. Overexpression of CBF-1 impaired WCC binding to the C-box to suppress frq transcription. Together, our results suggest that the proper level of CBF-1 is critical for circadian clock function by suppressing WC-independent FRQ expression and by regulating WCC binding to the frq promoter.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Suppression of WHITE COLLAR-independent frequency Transcription by Histone H3 Lysine 36 Methyltransferase SET-2 Is Necessary for Clock Function in Neurospora.J Biol Chem. 2016 May 20;291(21):11055-63. doi: 10.1074/jbc.M115.711333. Epub 2016 Mar 21. J Biol Chem. 2016. PMID: 27002152 Free PMC article.
-
Role for Protein Kinase A in the Neurospora Circadian Clock by Regulating White Collar-Independent frequency Transcription through Phosphorylation of RCM-1.Mol Cell Biol. 2015 Jun;35(12):2088-102. doi: 10.1128/MCB.00709-14. Epub 2015 Apr 6. Mol Cell Biol. 2015. PMID: 25848091 Free PMC article.
-
Transcriptional repression of frequency by the IEC-1-INO80 complex is required for normal Neurospora circadian clock function.PLoS Genet. 2017 Apr 12;13(4):e1006732. doi: 10.1371/journal.pgen.1006732. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28403234 Free PMC article.
-
The molecular workings of the Neurospora biological clock.Novartis Found Symp. 2003;253:184-98; discussion 102-9, 198-202, 281-4. Novartis Found Symp. 2003. PMID: 14712922 Review.
-
The neurospora circadian system.J Biol Rhythms. 2004 Oct;19(5):414-24. doi: 10.1177/0748730404269116. J Biol Rhythms. 2004. PMID: 15534321 Review.
Cited by
-
Regulation of the Neurospora Circadian Clock by the Spliceosome Component PRP5.G3 (Bethesda). 2019 Nov 5;9(11):3653-3661. doi: 10.1534/g3.119.400500. G3 (Bethesda). 2019. PMID: 31511298 Free PMC article.
-
The nutrient-sensing GCN2 signaling pathway is essential for circadian clock function by regulating histone acetylation under amino acid starvation.Elife. 2023 Apr 21;12:e85241. doi: 10.7554/eLife.85241. Elife. 2023. PMID: 37083494 Free PMC article.
-
STK-12 acts as a transcriptional brake to control the expression of cellulase-encoding genes in Neurospora crassa.PLoS Genet. 2019 Nov 25;15(11):e1008510. doi: 10.1371/journal.pgen.1008510. eCollection 2019 Nov. PLoS Genet. 2019. PMID: 31765390 Free PMC article.
-
The Frq-Frh Complex Light-Dependently Delays Sfl1-Induced Microsclerotia Formation in Verticillium dahliae.J Fungi (Basel). 2023 Jul 4;9(7):725. doi: 10.3390/jof9070725. J Fungi (Basel). 2023. PMID: 37504714 Free PMC article.
-
Differential regulation of phosphorylation, structure, and stability of circadian clock protein FRQ isoforms.J Biol Chem. 2023 Apr;299(4):104597. doi: 10.1016/j.jbc.2023.104597. Epub 2023 Mar 9. J Biol Chem. 2023. PMID: 36898580 Free PMC article.
References
-
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96(2):271–90. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

