Lso2 is a conserved ribosome-bound protein required for translational recovery in yeast
- PMID: 30208026
- PMCID: PMC6135351
- DOI: 10.1371/journal.pbio.2005903
Lso2 is a conserved ribosome-bound protein required for translational recovery in yeast
Abstract
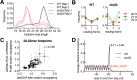
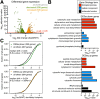
Ribosome-binding proteins function broadly in protein synthesis, gene regulation, and cellular homeostasis, but the complete complement of functional ribosome-bound proteins remains unknown. Using quantitative mass spectrometry, we identified late-annotated short open reading frame 2 (Lso2) as a ribosome-associated protein that is broadly conserved in eukaryotes. Genome-wide crosslinking and immunoprecipitation of Lso2 and its human ortholog coiled-coil domain containing 124 (CCDC124) recovered 25S ribosomal RNA in a region near the A site that overlaps the GTPase activation center. Consistent with this location, Lso2 also crosslinked to most tRNAs. Ribosome profiling of yeast lacking LSO2 (lso2Δ) revealed global translation defects during recovery from stationary phase with translation of most genes reduced more than 4-fold. Ribosomes accumulated at start codons, were depleted from stop codons, and showed codon-specific changes in occupancy in lso2Δ. These defects, and the conservation of the specific ribosome-binding activity of Lso2/CCDC124, indicate broadly important functions in translation and physiology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

