Perspectives for cancer immunotherapy mediated by p19Arf plus interferon-beta gene transfer
- PMID: 30208166
- PMCID: PMC6113850
- DOI: 10.6061/clinics/2018/e479s
Perspectives for cancer immunotherapy mediated by p19Arf plus interferon-beta gene transfer
Abstract
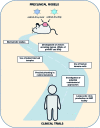
While cancer immunotherapy has gained much deserved attention in recent years, many areas regarding the optimization of such modalities remain unexplored, including the development of novel approaches and the strategic combination of therapies that target multiple aspects of the cancer-immunity cycle. Our own work involves the use of gene transfer technology to promote cell death and immune stimulation. Such immunogenic cell death, mediated by the combined transfer of the alternate reading frame (p14ARF in humans and p19Arf in mice) and the interferon-β cDNA in our case, was shown to promote an antitumor immune response in mouse models of melanoma and lung carcinoma. With these encouraging results, we are now setting out on the road toward translational and preclinical development of our novel immunotherapeutic approach. Here, we outline the perspectives and challenges that we face, including the use of human tumor and immune cells to verify the response seen in mouse models and the incorporation of clinically relevant models, such as patient-derived xenografts and spontaneous tumors in animals. In addition, we seek to combine our immunotherapeutic approach with other treatments, such as chemotherapy or checkpoint blockade, with the goal of reducing dosage and increasing efficacy. The success of any translational research requires the cooperation of a multidisciplinary team of professionals involved in laboratory and clinical research, a relationship that is fostered at the Cancer Institute of Sao Paulo.
Conflict of interest statement
No potential conflict of interest was reported.
Figures
Similar articles
-
Combined p14ARF and Interferon-β Gene Transfer to the Human Melanoma Cell Line SK-MEL-147 Promotes Oncolysis and Immune Activation.Front Immunol. 2020 Oct 22;11:576658. doi: 10.3389/fimmu.2020.576658. eCollection 2020. Front Immunol. 2020. PMID: 33193370 Free PMC article.
-
Harnessing combined p19Arf and interferon-beta gene transfer as an inducer of immunogenic cell death and mediator of cancer immunotherapy.Cell Death Dis. 2017 May 11;8(5):e2784. doi: 10.1038/cddis.2017.201. Cell Death Dis. 2017. PMID: 28492558 Free PMC article. No abstract available.
-
Combined p19Arf and interferon-beta gene transfer enhances cell death of B16 melanoma in vitro and in vivo.Cancer Gene Ther. 2013 May;20(5):317-25. doi: 10.1038/cgt.2013.23. Epub 2013 Apr 26. Cancer Gene Ther. 2013. PMID: 23618951
-
[Alternative protein p19ARF: a genuine tumor suppressor gene].Bull Cancer. 1998 Apr;85(4):304-6. Bull Cancer. 1998. PMID: 9752293 Review. French.
-
Immunotherapy and gene therapy as novel treatments for cancer.Colomb Med (Cali). 2017 Sep 30;48(3):138-147. doi: 10.25100/cm.v48i3.2997. Colomb Med (Cali). 2017. PMID: 29213157 Free PMC article. Review.
Cited by
-
p19Arf sensitizes B16 melanoma cells to interferon-β delivered via mesenchymal stem cells in vitro.Braz J Med Biol Res. 2020 Feb 14;53(3):e8876. doi: 10.1590/1414-431X20198876. eCollection 2020. Braz J Med Biol Res. 2020. PMID: 32077463 Free PMC article.
-
Perspectives for Combining Viral Oncolysis With Additional Immunotherapies for the Treatment of Melanoma.Front Mol Biosci. 2022 Apr 14;9:777775. doi: 10.3389/fmolb.2022.777775. eCollection 2022. Front Mol Biosci. 2022. PMID: 35495634 Free PMC article. Review.
-
Prognostic value of integrin αV expression and localization pattern in invasive breast carcinomas.Neoplasia. 2022 Aug;30:100803. doi: 10.1016/j.neo.2022.100803. Epub 2022 May 5. Neoplasia. 2022. PMID: 35526305 Free PMC article.
-
Combined p14ARF and Interferon-β Gene Transfer to the Human Melanoma Cell Line SK-MEL-147 Promotes Oncolysis and Immune Activation.Front Immunol. 2020 Oct 22;11:576658. doi: 10.3389/fimmu.2020.576658. eCollection 2020. Front Immunol. 2020. PMID: 33193370 Free PMC article.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144((5)):646–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

