Vibrational Control of Covalency Effects Related to the Active Sites of Molybdenum Enzymes
- PMID: 30208274
- PMCID: PMC7908822
- DOI: 10.1021/jacs.8b08254
Vibrational Control of Covalency Effects Related to the Active Sites of Molybdenum Enzymes
Abstract
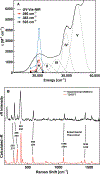
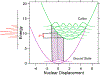
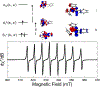
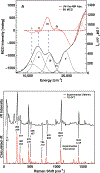
A multitechnique spectroscopic and theoretical study of the Cp2M(benzenedithiolato) (M = Ti, V, Mo; Cp = η5-C5H5) series provides deep insight into dithiolene electronic structure contributions to electron transfer reactivity and reduction potential modulation in pyranopterin molybdenum enzymes. This work explains the magnitude of the dithiolene folding distortion and the concomitant changes in metal-ligand covalency that are sensitive to electronic structure changes as a function of d-electron occupancy in the redox orbital. It is shown that the large fold angle differences correlate with covalency, and the fold angle distortion is due to a pseudo-Jahn-Teller (PJT) effect. The PJT effect in these and related transition metal dithiolene systems arises from the small energy differences between metal and sulfur valence molecular orbitals, which uniquely poise these systems for dramatic geometric and electronic structure changes as the oxidation state changes. Herein, we have used a combination of resonance Raman, magnetic circular dichroism, electron paramagnetic resonance, and UV photoelectron spectroscopies to explore the electronic states involved in the vibronic coupling mechanism. Comparison between the UV photoelectron spectroscopy (UPS) of the d2 M = Mo complex and the resonance Raman spectra of the d1 M = V complex reveals the power of this combined spectroscopic approach. Here, we observe that the UPS spectrum of Cp2Mo(bdt) contains an intriguing vibronic progession that is dominated by a "missing-mode" that is composed of PJT-active distortions. We discuss the relationship of the PJT distortions to facile electron transfer in molybdenum enzymes.
Figures


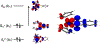



Similar articles
-
Resonance Raman spectroscopy of pyranopterin molybdenum enzymes.J Inorg Biochem. 2022 Oct;235:111907. doi: 10.1016/j.jinorgbio.2022.111907. Epub 2022 Jun 23. J Inorg Biochem. 2022. PMID: 35932756 Free PMC article. Review.
-
Understanding the origin of metal-sulfur vibrations in an oxo-molybdenum dithiolene complex: relevance to sulfite oxidase.Inorg Chem. 2006 Feb 6;45(3):967-76. doi: 10.1021/ic0506815. Inorg Chem. 2006. PMID: 16441102
-
Sulfur K-edge spectroscopic investigation of second coordination sphere effects in oxomolybdenum-thiolates: relationship to molybdenum-cysteine covalency and electron transfer in sulfite oxidase.Inorg Chem. 2007 Feb 19;46(4):1259-67. doi: 10.1021/ic061150z. Inorg Chem. 2007. PMID: 17291118
-
Electronic structure studies of oxomolybdenum tetrathiolate complexes: origin of reduction potential differences and relationship to cysteine-molybdenum bonding in sulfite oxidase.Inorg Chem. 2000 Dec 11;39(25):5697-706. doi: 10.1021/ic0003729. Inorg Chem. 2000. PMID: 11151370
-
Advancing Our Understanding of Pyranopterin-Dithiolene Contributions to Moco Enzyme Catalysis.Molecules. 2023 Nov 7;28(22):7456. doi: 10.3390/molecules28227456. Molecules. 2023. PMID: 38005178 Free PMC article. Review.
Cited by
-
Spectroscopic Studies of Mononuclear Molybdenum Enzyme Centers.Molecules. 2022 Jul 27;27(15):4802. doi: 10.3390/molecules27154802. Molecules. 2022. PMID: 35956757 Free PMC article. Review.
-
Resonance Raman spectroscopy of pyranopterin molybdenum enzymes.J Inorg Biochem. 2022 Oct;235:111907. doi: 10.1016/j.jinorgbio.2022.111907. Epub 2022 Jun 23. J Inorg Biochem. 2022. PMID: 35932756 Free PMC article. Review.
-
Protonation and Non-Innocent Ligand Behavior in Pyranopterin Dithiolene Molybdenum Complexes.Inorg Chem. 2022 Sep 5;61(35):13728-13742. doi: 10.1021/acs.inorgchem.2c01234. Epub 2022 Aug 24. Inorg Chem. 2022. PMID: 36000991 Free PMC article.
-
Second-Coordination-Sphere Effects Reveal Electronic Structure Differences between the Mitochondrial Amidoxime Reducing Component and Sulfite Oxidase.Inorg Chem. 2024 Oct 14;63(41):19063-19073. doi: 10.1021/acs.inorgchem.4c02157. Epub 2024 Sep 30. Inorg Chem. 2024. PMID: 39350518
-
Molybdenum Cofactor Model Reveals Remarkable Redox Activity at Both Molybdenum and the Pyranopterin Dithiolene Ligand.J Am Chem Soc. 2025 May 7;147(18):15088-15099. doi: 10.1021/jacs.4c17577. Epub 2025 Apr 27. J Am Chem Soc. 2025. PMID: 40289353 Free PMC article.
References
-
- McCleverty JA, Metal 1,2-Dithiolene and Related Complexes. Prog. Inorg. Chem 1968, 10, 49–221.
-
- Stiefel EI, Dithiolene Chemistry: Synthesis, Properties, and Applications. John Wiley and Sons, Inc: Hoboken, New Jersey, 2003; Vol. 52.
-
- Eisenberg R; Gray HB, Noninnocence in Metal Complexes: A Dithiolene Dawn. lnorg. Chem 2011, 50, 9741–9751. - PubMed
-
- Kirk ML; McNaughton RL; Helton ME, The Electronic Structure and Spectroscopy of Metallo-Dithiolene Complexes. In Progress in Inorganic Chemistry: Synthesis, Properties, and Applications, Stiefel EI; Karlin KD, Eds. John Wiley and Sons: Hoboken, New Jersey, 2004; Vol. 52, pp 111–212.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

