Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder
- PMID: 30208470
- PMCID: PMC6218311
- DOI: 10.1001/jamapediatrics.2018.2143
Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder
Abstract
Importance: Retention in addiction treatment is associated with reduced mortality for individuals with opioid use disorder (OUD). Although clinical trials support use of OUD medications among youths (adolescents and young adults), data on timely receipt of buprenorphine hydrochloride, naltrexone hydrochloride, and methadone hydrochloride and its association with retention in care in real-world treatment settings are lacking.
Objectives: To identify the proportion of youths who received treatment for addiction after diagnosis and to determine whether timely receipt of OUD medications is associated with retention in care.
Design, setting, and participants: This retrospective cohort study used enrollment data and complete health insurance claims of 2.4 million youths aged 13 to 22 years from 11 states enrolled in Medicaid from January 1, 2014, to December 31, 2015. Data analysis was performed from August 1, 2017, to March 15, 2018.
Exposures: Receipt of OUD medication (buprenorphine, naltrexone, or methadone) within 3 months of diagnosis of OUD compared with receipt of behavioral health services alone.
Main outcomes and measures: Retention in care, with attrition defined as 60 days or more without any treatment-related claims.
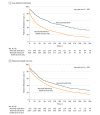
Results: Among 4837 youths diagnosed with OUD, 2752 (56.9%) were female and 3677 (76.0%) were non-Hispanic white. Median age was 20 years (interquartile range [IQR], 19-21 years). Overall, 3654 youths (75.5%) received any treatment within 3 months of diagnosis of OUD. Most youths received only behavioral health services (2515 [52.0%]), with fewer receiving OUD medications (1139 [23.5%]). Only 34 of 728 adolescents younger than 18 years (4.7%; 95% CI, 3.1%-6.2%) and 1105 of 4109 young adults age 18 years or older (26.9%; 95% CI, 25.5%-28.2%) received timely OUD medications. Median retention in care among youths who received timely buprenorphine was 123 days (IQR, 33-434 days); naltrexone, 150 days (IQR, 50-670 days); and methadone, 324 days (IQR, 115-670 days) compared with 67 days (IQR, 14-206 days) among youths who received only behavioral health services. Timely receipt of buprenorphine (adjusted hazard ratio, 0.58; 95% CI, 0.52-0.64), naltrexone (adjusted hazard ratio, 0.54; 95% CI, 0.43-0.69), and methadone (adjusted hazard ratio, 0.32; 95% CI, 0.22-0.47) were each independently associated with lower attrition from treatment compared with receipt of behavioral health services alone.
Conclusions and relevance: Timely receipt of buprenorphine, naltrexone, or methadone was associated with greater retention in care among youths with OUD compared with behavioral treatment only. Strategies to address the underuse of evidence-based medications for youths with OUD are urgently needed.
Conflict of interest statement
Figures
Comment in
-
Medication assisted therapy (MAT) for opioid use disorder (OUD) in youth improves outcomes and saves lives.Evid Based Nurs. 2020 Jul;23(3):77. doi: 10.1136/ebnurs-2018-103053. Epub 2019 Aug 28. Evid Based Nurs. 2020. PMID: 31462425 No abstract available.
References
-
- Curtin SC, Tejada-Vera B, Warner M Drug overdose deaths among adolescents aged 15–19 in the United States: 1999-2015. NCHS data brief no 282. Hyattsville, MD: Centers for Disease Control and Prevention; 2017. - PubMed
-
- Zibbell JE, Iqbal K, Patel RC, et al. ; Centers for Disease Control and Prevention (CDC) . Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453-458. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical