Autophagy regulates glucose-mediated root meristem activity by modulating ROS production in Arabidopsis
- PMID: 30208757
- PMCID: PMC6351127
- DOI: 10.1080/15548627.2018.1520547
Autophagy regulates glucose-mediated root meristem activity by modulating ROS production in Arabidopsis
Abstract
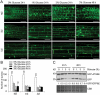
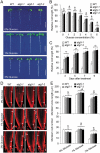
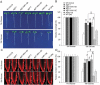
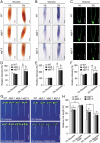
Glucose produced from photosynthesis is a key nutrient signal regulating root meristem activity in plants; however, the underlying mechanisms remain poorly understood. Here, we show that, by modulating reactive oxygen species (ROS) levels, the conserved macroautophagy/autophagy degradation pathway contributes to glucose-regulated root meristem maintenance. In Arabidopsis thaliana roots, a short exposure to elevated glucose temporarily suppresses constitutive autophagosome formation. The autophagy-defective autophagy-related gene (atg) mutants have enhanced tolerance to glucose, established downstream of the glucose sensors, and accumulate less glucose-induced ROS in the root tips. Moreover, the enhanced root meristem activities in the atg mutants are associated with improved auxin gradients and auxin responses. By acting with AT4G39850/ABCD1 (ATP-binding cassette D1; Formerly PXA1/peroxisomal ABC transporter 1), autophagy plays an indispensable role in the glucose-promoted degradation of root peroxisomes, and the atg mutant phenotype is partially rescued by the overexpression of ABCD1. Together, our findings suggest that autophagy is an essential mechanism for glucose-mediated maintenance of the root meristem. Abbreviation: ABA: abscisic acid; ABCD1: ATP-binding cassette D1; ABO: ABA overly sensitive; AsA: ascorbic acid; ATG: autophagy related; CFP: cyan fluorescent protein; Co-IP: co-immunoprecipitation; DAB: 3',3'-diaininobenzidine; DCFH-DA: 2',7'-dichlorodihydrofluorescin diacetate; DR5: a synthetic auxin response element consists of tandem direct repeats of 11 bp that included the auxin-responsive TGTCTC element; DZ: differentiation zone; EZ, elongation zone; GFP, green fluorescent protein; GSH, glutathione; GUS: β-glucuronidase; HXK1: hexokinase 1; H2O2: hydrogen peroxide; IAA: indole-3-acetic acid; IBA: indole-3-butyric acid; KIN10/11: SNF1 kinase homolog 10/11; MDC: monodansylcadaverine; MS: Murashige and Skoog; MZ: meristem zone; NBT: nitroblue tetrazolium; NPA: 1-N-naphtylphthalamic acid; OxIAA: 2-oxindole-3-acetic acid; PIN: PIN-FORMED; PLT: PLETHORA; QC: quiescent center; RGS1: Regulator of G-protein signaling 1; ROS: reactive oxygen species; SCR: SCARECROW; SHR, SHORT-ROOT; SKL: Ser-Lys-Leu; SnRK1: SNF1-related kinase 1; TOR: target of rapamycin; UPB1: UPBEAT1; WOX5: WUSCHEL related homeobox 5; Y2H: yeast two-hybrid; YFP: yellow fluorescent protein.
Keywords: Autophagy; glucose; peroxisome; reactive oxygen species; root meristem.
Figures


Similar articles
-
ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis.PLoS Genet. 2014 Dec 18;10(12):e1004791. doi: 10.1371/journal.pgen.1004791. eCollection 2014 Dec. PLoS Genet. 2014. PMID: 25522358 Free PMC article.
-
A facile forward-genetic screen for Arabidopsis autophagy mutants reveals twenty-one loss-of-function mutations disrupting six ATG genes.Autophagy. 2019 Jun;15(6):941-959. doi: 10.1080/15548627.2019.1569915. Epub 2019 Feb 8. Autophagy. 2019. PMID: 30734619 Free PMC article.
-
Involvement of reactive oxygen species and auxin in serotonin-induced inhibition of primary root elongation.J Plant Physiol. 2018 Oct;229:89-99. doi: 10.1016/j.jplph.2018.07.004. Epub 2018 Jul 18. J Plant Physiol. 2018. PMID: 30055520
-
Hydrogen Peroxide Signaling in the Maintenance of Plant Root Apical Meristem Activity.Antioxidants (Basel). 2024 Apr 30;13(5):554. doi: 10.3390/antiox13050554. Antioxidants (Basel). 2024. PMID: 38790659 Free PMC article. Review.
-
Modulatory Role of Reactive Oxygen Species in Root Development in Model Plant of Arabidopsis thaliana.Front Plant Sci. 2020 Sep 16;11:485932. doi: 10.3389/fpls.2020.485932. eCollection 2020. Front Plant Sci. 2020. PMID: 33042167 Free PMC article. Review.
Cited by
-
Regulation of Superoxide by BAP31 through Its Effect on p22phox and Keap1/Nrf2/HO-1 Signaling Pathway in Microglia.Oxid Med Cell Longev. 2021 Mar 9;2021:1457089. doi: 10.1155/2021/1457089. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33777312 Free PMC article.
-
Duck Tembusu virus infection induces mitochondrial-mediated and death receptor-mediated apoptosis in duck embryo fibroblasts.Vet Res. 2022 Jul 7;53(1):53. doi: 10.1186/s13567-022-01070-9. Vet Res. 2022. PMID: 35799206 Free PMC article.
-
Acetylation modification in the regulation of macroautophagy.Adv Biotechnol (Singap). 2024 Jun 7;2(2):19. doi: 10.1007/s44307-024-00027-7. Adv Biotechnol (Singap). 2024. PMID: 39883319 Free PMC article. Review.
-
Enhanced Autophagic Activity Improved the Root Growth and Nitrogen Utilization Ability of Apple Plants under Nitrogen Starvation.Int J Mol Sci. 2021 Jul 28;22(15):8085. doi: 10.3390/ijms22158085. Int J Mol Sci. 2021. PMID: 34360850 Free PMC article.
-
Role of autophagy in plant growth and adaptation to salt stress.Planta. 2025 Jan 31;261(3):49. doi: 10.1007/s00425-025-04615-2. Planta. 2025. PMID: 39885016 Review.
References
-
- Sheen J, Zhou L, Jang J.. Sugars as signaling molecules. Curr Opin Plant Biol. 1999;2(5):410–418. - PubMed
-
- Leon P. Sugar and hormone connections. Trends Plant Sci. 2003;8(3):110–116. - PubMed
-
- Gibson SI, Sugar and phytohormone response pathways: navigating a signalling network. J Exp Bot. 2004;55(395):253–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
