Low pH-responsive proteins revealed by a 2-DE based MS approach and related physiological responses in Citrus leaves
- PMID: 30208853
- PMCID: PMC6134590
- DOI: 10.1186/s12870-018-1413-3
Low pH-responsive proteins revealed by a 2-DE based MS approach and related physiological responses in Citrus leaves
Abstract
Background: Rare data are available on the molecular responses of higher plants to low pH. Seedlings of 'Sour pummelo' (Citrus grandis) and 'Xuegan' (Citrus sinensis) were treated daily with nutrient solution at a pH of 2.5, 3, or 6 (control) for nine months. Thereafter, we first used 2-dimensional electrophoresis (2-DE) to investigate low pH-responsive proteins in Citrus leaves. Meanwhile, we examined low pH-effects on leaf gas exchange, carbohydrates, ascorbate, dehydroascorbate and malondialdehyde. The objectives were to understand the adaptive mechanisms of Citrus to low pH and to identify the possible candidate proteins for low pH-tolerance.
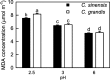
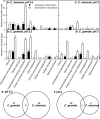
Results: Our results demonstrated that Citrus were tolerant to low pH, with a slightly higher low pH-tolerance in the C. sinensis than in the C. grandis. Using 2-DE, we identified more pH 2.5-responsive proteins than pH 3-responsive proteins in leaves. This paper discussed mainly on the pH 2.5-responsive proteins. pH 2.5 decreased the abundances of proteins involved in ribulose bisphosphate carboxylase/oxygenase activation, Calvin cycle, carbon fixation, chlorophyll biosynthesis and electron transport, hence lowering chlorophyll level, electron transport rate and photosynthesis. The higher oxidative damage in the pH 2.5-treated C. grandis leaves might be due to a combination of factors including higher production of reactive oxygen species, more proteins decreased in abundance involved in antioxidation and detoxification, and lower ascorbate level. Protein and amino acid metabolisms were less affected in the C. sinensis leaves than those in the C. grandis leaves when exposed to pH 2.5. The abundances of proteins related to jasmonic acid biosynthesis and signal transduction were increased and decreased in the pH 2.5-treated C. sinensis and C. grandis leaves, respectively.
Conclusions: This is the first report on low pH-responsive proteins in higher plants. Thus, our results provide some novel information on low pH-toxicity and -tolerance in higher plants.
Keywords: 2-DE; Citrus grandis; Citrus sinensis; Leaves; Low pH; Proteomics.
Conflict of interest statement
Ethics approval and consent to participate
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. The author Li-Song Chen is an Associate Editor of BMC Plant Biology.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. doi: 10.1007/BF00009558. - DOI
-
- Shi QH, Zhu ZJ, Juan LI, Qian QQ. Combined effects of excess Mn and low pH on oxidative stress and antioxidant enzymes in cucumber roots. Agri Sci China. 2006;5:767–772. doi: 10.1016/S1671-2927(06)60122-3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

